α-oxidation
The α-oxidation is an alternative biochemical breakdown mechanism for fatty acids , which is used when the normally used β-oxidation is impossible. This is the case when a methyl group at Cβ prevents its third step, oxidation to the ketone. The name α-oxidation describes that the carbon atom adjacent to the carboxy group ( α-position ) is oxidized .
In mammals , phytanic acid is the only fatty acid to break down in this way. This is created by oxidation from phytol , an alcohol that is found in esterified form in chlorophyll . Unlike humans, ruminants are able to break down this ester during passage through the intestine; About 100 mg enter the human body daily through their meat and milk . From an energetic point of view, α-oxidation only plays a subordinate role.
Step-by-step process
activation
Before the breakdown can begin, the acid has to be bound to coenzyme A. In order to provide the necessary energy for the formation of the thioester , pyrophosphate is split off from ATP . This reaction is catalyzed by a phytanoyl-CoA ligase ( EC 6.2.1.24 ):
Degradation in the peroxisomes
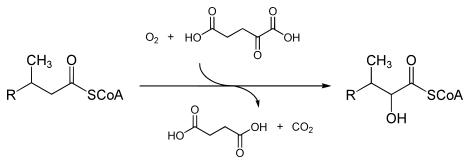
The enzyme peroxisomal phytanoyl-CoA dioxygenase ( EC 1.14.11.18 ) oxidizes the α-C atom of phytanic acid in the presence of ascorbic acid and iron with molecular oxygen to form alcohol - the second oxygen atom is transferred to 2-oxoglutarate :
2-Hydroxyacyl-CoA lyase 1 catalyzes the cleavage into formyl-CoA and pristanal ; Cofactors are Mg 2+ and thiamine pyrophosphate :
Hydrogen is split off from the aldehyde , or, more precisely: from its hydrate , by the enzyme pristanal dehydrogenase and transferred to NAD⁺:
Very long chain acyl CoA synthetase reactivates the acid; the energy comes from ATP:
This reaction is necessary in order to transport the pristanic acid produced from the peroxisomes back into the cytoplasm - it can be completely broken down in the mitochondria by β-oxidation to 3 acetyl-CoA , 3 propionyl-CoA and isobutyryl-CoA .
Pathobiochemistry
If either the enzyme peroxisomal phytanoyl-CoA dioxygenase , which catalyzes the hydroxylation at Cα, or peroxin-7 , a protein that transports this enzyme into the peroxisomes , is defective, phytanic acid cannot be broken down. As a result, it accumulates in the body and causes a condition called Refsum's syndrome with severe neurological problems. Permanent cure does not exist, by a phytansäurearme diet the symptoms of the disease can go back again.
Individual evidence
- ↑ a b Thomas M. Devlin (Ed.): Textbook of Biochemistry with Clinical Correlations . Wiley & Sons; 6th edition 2006; ISBN 978-0-471-67808-3 ; P. 686
- ↑ UniProt O14832
- ↑ UniProt Q9UJ83
- ↑ not yet recorded
- ↑ UniProt O14975
See also
literature
- Gerbert A. Jansen, Ronald JA Wanders: Alpha Oxidation. In: Biochimica et Biophysica Acta - Molecular Cell Research. 1763, 2006, p. 1403, doi : 10.1016 / j.bbamcr.2006.07.012 .
Web links
- Anthony S. Wierzbicki (2007): Peroxisomal disorders affecting phytanic acid alpha-oxidation: a review . In: Biochem Soc Trans . 35 (Pt 5); 881-886; ISSN 0300-5127 PMID 17956237 ; PDF (free full text access)
- Ronald J. Wanders and Jasper C. Komen (2007): Peroxisomes, Refsum's disease and the alpha- and omega-oxidation of phytanic acid . In: Biochem Soc Trans . 35 (Pt 5); 865-869; ISSN 0300-5127 PMID 1795623 ; PDF (free full text access)