Trimeperidine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
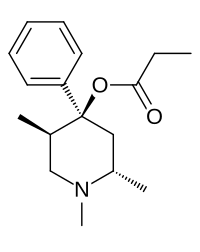
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimeperidine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 25 NO 2 | |||||||||||||||
| Brief description |
white, crystalline powder with a bitter taste |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 275.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
soluble in water and chloroform, insoluble in acetone and benzene |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trimeperidin (Promedol) is an opioid analgesic and an analog of Prodin. It was developed in the USSR in the early 1950s during research into the related substance pethidine .
Trimeperidine has four structural isomers, two of which are pharmacologically active, the γ-isomer trimeperidine and the β-isomer isopromedol. It has about half the analgesic potency of morphine and has been used extensively as a pain reliever.
Trimeperidine produces effects similar to other opioids , such as analgesia and sedation , along with side effects such as nausea , itching and hypoventilation that can be harmful to fatal.
Trimeperidine is classified as a Class I drug in the Controlled Substances Act classification as an addictive substance . It is listed in the standard convention on narcotics and is subject to similar restrictions as morphine or heroin in most countries .
Individual evidence
- ↑ a b c P. H. List, L. Hörhammer: General part. Active ingredient groups I (= Handbook of Pharmaceutical Practice ). 4th edition. Springer-Verlag, Berlin / Heidelberg 2013, ISBN 978-3-642-47985-4 , p. 797 , doi : 10.1007 / 978-3-642-47985-4_18 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ IN Nazarov, NS Prostakov, NI Shvetsov: Russian Journal of General Chemistry. Volume 26, 1956, p. 2798.
- ↑ AF Casy, K. McErlane: Analgesic potency and stereochemistry of trimeperidine and its isomers and analogues . In: Journal of Pharmacy and Pharmacology . tape 23 , no. 1 , January 1971, p. 68-69 , doi : 10.1111 / j.2042-7158.1971.tb12786.x .
- ^ EN Guseva: [Comparative analgesic effects of promedol, phenadone, tecodine, and morphine] . In: Farmakologiia I Toksikologiia . tape 19 , Suppl, January 1956, pp. 17-18 , PMID 13448009 .
- ↑ KI Bender, OV Gerasimova: [Relationship between the pain-relieving action of narcotic analgesics and their effect on respiration] . In: Farmakologiia I Toksikologiia . tape 39 , no. 5 , October 1976, p. 552-556 , PMID 18367 .
- ↑ EA Chernukha, NN Rasstrigin: [Anesthesia in labor] . In: Fel ʹ dsher I Akusherka . tape 45 , no. January 6 , 1980, pp. 21-27 , PMID 6901667 .
- ↑ Iu V. Zhirkova, SM Stepanenko, O. Iu Butyleva, EV Zilbert, AF Manerova, NV Golodenko: [Method of continuous intravenous postoperative analgesia with promedol in newborn children] . In: Anesteziologiia I Reanimatologiia . No. 1 , February 2004, p. 12-16 , PMID 15206301 .
- ↑ Final Adjusted Aggregate Production Quotas for Schedule I and II Controlled Substances and Assessment of Annual Needs for the List I Chemicals Ephedrine, Pseudoephedrine, and Phenylpropanolamine for 2014. DEA , accessed September 11, 2016 .