Respiration measurement
A respiration measurement examines the breathing of a single living being or a group of living beings. The respiration measurement is used in the energetic evaluation of feed and rations as well as in the determination of the energy requirements of farm animals for the various services. In humans, with the respiration measurement metabolism examinations z. B. to research disorders such as obesity or during sporting activities.
history
The energy consumption in the carcass can be determined directly through control slaughter, in that the energy content and material composition of an animal group are determined at the beginning ( control group ) and at the end of the growth phase (test group), provided that the animal experiments are limited to the energy requirements for growth . The data from the zero group are transferred to the test group at the beginning of the growing season. For the indirect measurement of the energy turnover of humans and animals, facilities for determining the heat are required. These were mainly developed in human medicine and animal nutrition research. In human medicine, metabolic balances were described by Max von Pettenkofer in collaboration with Carl von Voit around 1860 . In animal nutrition research, Gustav Kühn , Oskar Kellner and Gustav Fingerling carried out total metabolism tests with various types of farm animals in the agricultural research station in Möckern near Leipzig from 1880 to 1940 .
Measuring systems
In direct calorimetry , as z. B. by Armsby and Rubner was used, the heat production is measured directly by placing the test animal in a calorimeter. The animal experiment chamber is completely surrounded by a water jacket, from the change in temperature of which it is possible to infer the heat given off by the animal. The indirect calorimetry (respiratory calorimetry), as z. B. by Pettenkofer, Kühn and Kellner was used, determines the heat production indirectly, d. H. calculated using the gas exchange data of the test animal. The gas exchange can be measured according to two principles. With the closed principle of gas exchange measurement ( Regnault and Reise), the exhaust air from the respiration chamber is processed and then returned to it. With the open principle of gas exchange measurement (by Pettenkofer and Voit), fresh air is introduced into the respiration chamber, the exhaust air is measured and analyzed and then released into the atmosphere. The exhaust air, the actual test air, is analyzed in aliquots using the Pettenkofer's principle, after the exact volume has been recorded. A modification of Pettenkofer's principle goes back to Haldane. According to Haldane, the carbon dioxide and the oxidation water are absorbed by suitable reagents from the total amount of gas passed through and recorded gravimetrically .
Construction of the respiratory systems for large animals
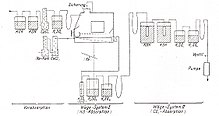
In the Oskar-Kellner-Institut in Rostock, ten respiratory systems for large animals (cattle, pigs, sheep) were built according to the open principle between 1955 and 1957, four each for cattle and pigs and two for sheep. A system can be divided into three main components (Fig. 1). The animal is in the air-conditioned respiration chamber 1). With the help of the respiration pump 2), fresh air is sucked into the chamber through the fresh air duct 1a) and sucked off via the test air duct 1b) after passing through the gas mixer 2a) and humidification tower 2b) and pressed into the atmosphere on the side of the building opposite the fresh air supply. The air volume is also measured with the respiration pump. It can also be used to vary the gas throughput, namely between 250 and 500 m³ / day for the equipment for cattle, between 70 and 140 m³ / day for those for pigs and between 35 and 70 m³ / day for those for sheep. The gas sample pumps 3a1) - 3a4) work in the same direction as the respiration pump. Two of them suck in parallel samples of the test air, one sucks in a sample of fresh air and the fourth pump is used to operate the gas meter for driving the recipients. The spirometers 3b) convert the discontinuous sample gas flow into a continuous flow. For each respiration experiment, two test air samples of 3–5 liters are drawn continuously from the recipients for the analysis over the 24 hours of the experiment. The gas quantity measurement by the respiration pump takes place at a water vapor saturation of 100%, produced by the humidification tower. The temperature of the gas flow is recorded by a temperature recorder. The atmospheric pressure is recorded by pressure recorders, since the respiratory pumps work practically at atmospheric pressure. The data for the reduction of the gas volume to normal conditions are thus available. All 10 respiration systems are built according to this basic scheme.
In order to do justice to the herd feeling of the cattle, two chambers are assembled so that the animals can see each other through two gas-tight windows. The volume of the chamber is 9.2 m³. 4 windows are fitted gas-tight on the outer wall of the chamber. The chambers are made of 5 mm thick, galvanized sheet iron. The excrement can easily be removed from below the chamber. The fresh air is supplied over the entire width of the front side by means of a channel provided with holes. A plug valve is inserted into the pipeline 1a) with which a negative pressure of approx. 10 mm water column is set in the chamber, which sucks in a safety bell 4a) which is immersed in a channel above the feed sluice 5a) that is filled with water ( Liquid seal). When the air flow is interrupted, the safety bell is raised by a counterweight 4c) via a lever guide 4b) and the test animal is supplied with room air (in 40 years of intensive testing, no animal suffocated in a respiration chamber). The chambers contain an air conditioning system with a fan wheel 3a), which sucks in the air above the animal and leads it to dehumidification via the cooling system 3b) and then via the heating system 3c). The temperature in the chamber can be automatically regulated to 3–35 ± 1 ° C by means of a relay circuit. The feed sluice 5) is coupled to the safety device. The feed trough 5a) can be brought from the outside hydraulically 5b) into the feeding position 5a1) and into the loading position 5a2). In position 5a1) the animal has access to the feed, in position 5a2) the trough is pressed against a rubber seal to seal it and can be filled with feed. In this position the safety device does not work, the trough must be in position 5a1) or halfway between the two positions if the safety device is to be in action. The antechamber 2), which could be used to calibrate the respiratory pump system, was not required because the entire system could be calibrated without any problems. The built-in milking parlor 8) has not proven itself. For milking the cows, the milker with the milking cluster was granted access to the chamber by a second person under standardized conditions for a correction of the gas exchange. The milk was sucked into the milk can outside the chamber using a can milking machine through a valve that the second person opened. Today the milker enters the chamber with a gas mask through a lock (see below). The chamber door hangs on 2 hinges and is pressed against a rubber seal located in a U-rail by means of 6 eccentric levers (cam 10).
The respiration chambers for pigs, which can also be used for calves or groups of piglets, have a volume of 1.8 m³, those for sheep of 1.0 m³. In principle, they correspond in structure to the cattle chamber.
Construction of the respiratory systems for small animals
In the Oskar-Kellner-Institut numerous respiration experiments with small animals (rats as model animals for monogastrids , rabbits and chickens) were carried out. The Haldane principle for gas exchange measurement is best suited for these types of animals , because the carbon dioxide production of the animals is recorded with little effort, so no sampling systems are required. The use of chemicals is limited with this principle, but it can only be used for small animals, where it was the method of choice until about 20 years ago.
Weighing systems I and II are weighed with special scales (for rabbits with 50 or 30 kg load capacity, 100 mg sensitivity). The hanging rubber hose connections between the systems allow uninfluenced weighing while the test is running. The respiration box is completely gas-tight. It has a volume of 80 liters. The supply air, due to the pre-absorption of water and CO 2 -free outside air, enters via a tube that is guided over the width of the box and provided with holes on the upper face of the box. The exhaust air is extracted in a corresponding manner at the lower rear. Windows are inserted gas-tight at the front and on the side walls. The feed vessel, urine funnel and the excrement strainer on top can be changed at short notice by loosening a bracket screw connection. The feed vessel is divided for feed and drinking water. A safety device is attached to the respiration box, which consists of a bell that hangs on a helical spring and dips into a channel filled with mercury as a sealing liquid. This is necessary because of the resistance in the absorption system of around 15 mm of mercury . When the pump is running, the safety bell is sucked in; if the pump fails, the pressure is slowly equalized via the pre-absorption vessels and the remaining lower negative pressure is overcome by the spring and the animal is supplied with room air via the opening that has become free. The gas throughput is brought about by an eccentric vacuum pump, the throughput level of which can be regulated via an interposed pinch valve (Fig. 3). The air flow first passes the pre-absorption (see above) and enters the respiration box free of water and CO 2 . Here the animal absorbs the required amounts of oxygen and excretes CO 2 and water vapor. This water vapor, including the water vapor carried along with the air stream from drinking water and urine, is stored in the absorption battery connected downstream of the respiration box by means of conc. Sulfuric acid absorbed quantitatively. Together with the respiration box, this forms the weighing system I. In the bottle with dilute sulfuric acid, the small amounts of ammonia from the respiration box are quantitatively absorbed and can be determined. After passing through the weighing system I, the anhydrous but carbon dioxide-containing gas stream is sucked through the weighing system II. A gas filter frit is installed in each of the two absorption bottles filled with 20% potassium hydroxide solution for fine distribution of the gas flow and thus for achieving quantitative absorption of the CO 2 . In the last two absorption bottles with conc. Sulfuric acid, the water vapor carried along with the air stream from the potassium hydroxide solution is absorbed. The weight increase of the weighing system II gives the total CO 2 production of the animal. Since oxygen is the only reactive substance in the organism that is supplied to the entire system during the respiration experiment, the increase in weight of weighing systems I and II means the animal's oxygen consumption.
Gas analysis methods
With the further development of gas analysis , the question of the importance and usefulness of the systems and principles of the respiratory apparatus arises. In particular, the Haldane principle can no longer be seen as the method of choice for small animals, as can be seen in the next section. The gas analysis by binding the gases to chemicals was carried out with an Orsat device modified by Carpenter, Lee and Finnerty (2). The change was due to bulges in the measuring burette outside the measuring range. The measuring ranges in respiratory gases were between 100 and 98.4% for CO 2 and between 79.6 and 78.4% for oxygen. This increased the measurement and reading accuracy. CO 2 was bound to potassium hydroxide solution and the reduction in volume was read off. Methane was oxidized by glowing the gas on a platinum wire and determined to be CO 2 . Oxygen was reduced with pyrogallol . Because of the problems with pyrogallol, Schiemann switched to RCh-O 2 reducing agents and made modifications to the apparatus by Carpenter, Lee and Finnerty (2). One change was to make work easier by moving the gas between the absorption pipette and the measuring burette using a vacuum rather than by hand. Another related to fine-tuning the menisci using a pinch cock .
Although the time required for each gas analysis was reduced, it was very high overall. The switch from chemical to physical gas analysis leads to a significant reduction in the time required, which is only minutes. The accuracy of the analysis also increases. In the Oskar-Kellner-Institut the conversion could take place in 1962. By coupling “URAS devices” (ultrared absorption recorders) with dial galvanometers, the reading accuracy is increased. In the case of the polyatomic molecules CO 2 and CH 4 , the measuring principle is based on the intensified and concentration-dependent absorption of light in the infrared spectral range. The O 2 concentration can be measured based on the specific paramagnetism .
New facilities for continuous respiration measurements
At the new location of the "Oskar-Kellner" nutritional physiology research area of the Leibniz Institute for Farm Animal Biology ( FBN ) in Dummerstorf near Rostock, four modern respiratory systems were installed. In doing so, it was possible to fall back on the experience and further development of the devices at the old location, as described above. In front of the respiration chambers there is a scale on which the animals are weighed before entering and after leaving the chamber. The ventilation system is shown schematically in Figure 4.
The four chambers with dimensions of 4 × 2 × 2 m are set up in pairs so that two animals can see each other through the partition made of acrylic glass. The chamber doors can be closed tightly, as described above. The manger is 1 m wide, 1 m high and 0.5 m deep. The feed consumption is constantly recorded in each chamber via a scale and electronic registration. The water absorption is measured with a water meter and registered electronically. The standing and lying times are registered photoelectrically. Further physical activities are recorded with a modified infrared motion detector. The behavior of the animals is observed with an infrared reflector and camera, which are connected to computers. Observation is also possible by the employees from the apartment. An alarm system informs about the failure of the fresh air supply. At the front of each chamber there is a lock, the gas concentration of which corresponds to that of the chamber and through which access to the chamber (milking the cows or other activities) is possible without influencing the test air. The employee wears a gas mask with a connection to the outside while in the chamber. Blood can be taken from animals with catheters from the outside, mainly from the lock, without having to enter the chamber.
The chambers can be air-conditioned in the temperature range of 0–35 ° C and in the range for relative humidity of 50–70%. The air is sucked through the chamber with a vacuum pump with a capacity of 40 m³ / h. The air flow rate can be varied from 0–30 m³ / h using a bypass. This possibility of variation is not only important for the different feeding intensity of the cattle and the following variation in the gas exchange. The respiratory systems can also be used for experiments with calves, pigs and other animal categories of a similar size. For this purpose, a metabolism box adapted to the size of the animal is placed in the respiration chamber and connected to the corresponding air lines. This adjusts the volume to the needs. The air flow is recorded quantitatively with a flow meter based on differential pressure. The test air sample for the gas analysis is drawn with a membrane pump (80 l / h) about 10 cm after the flow meters. The sample air then passes through the analyzers to determine the CO 2 and CH 4 content on the basis of the infrared absorption and the O 2 content using its paramagnetism. There are duplicate gas analyzers so that there is no failure of the experiment due to a defective device. If a respiration chamber is used, the gas analysis takes place every 10 s; if all 4 chambers are used, the gas concentration is measured every 6 minutes. An outside air sample is included in each cycle in order to detect any drift in the analyzers.
All measurement data (gas concentrations of CO 2 , CH 4 and O 2 , the air flow rate, feed and water consumption, air temperature and relative humidity in and after the chamber, air pressure , standing and lying time as well as change of position and animal weight) are transferred to a storage system ( Simatic , Siemens) and collected with a purpose-adapted software ( WinCC , version 5.1, SP 2, Siemens). A software was developed for the automatic calculation of heat production (H. Scholze, FBN Dummerstorf). The program is based on Delphi (Delphi 2007, San Francisco). The calculation of the heat production (WP) is based on Brouwer:
- WP (kJ) = 16.18 O 2 (L) + 5.02 CO 2 (L) - 2.17 CH 4 (L) - 5.99 N (g).
At the new location of the research area there are also 6 respiratory devices for mice that work according to the system described above. With this system, the gas exchange can be measured with the gas analyzers mentioned in a short time. It should be particularly emphasized that the measurements are carried out continuously and thus enable better insights into the physiology of the metabolism . By a rhinomanometry the air resistance of the nasal cavity can be determined.
literature
- TM Carpenter, R. Lee, AE Finnerty: An apparatus for the exact and rapid analysis of gas from a respiration chamber. In: Wiss. Arch. Agriculture. 4 (1930) 1, Dept. B.
- M. Derno, H.-G. Elsner, E.-A. Paetow, H. Scholze, M. Schweigel: Technical note: A new facility for continuous respiration measurements in lactating cows. In: J. Dairy Sci. 92 (2009), pp. 2804-2808.
- K. Nehring, R. Schiemann, L. Hoffmann, M. Schmidt: About the construction of respiratory systems for cattle, pigs and sheep in the context of the new building of the Oskar-Kellner-Institute for Animal Nutrition. In: Rostock. Knowledge Abh. DAL, Berlin 37 (1958), pp. 34-64.
- R. Schiemann, R., L. Hoffmann, M. Schmidt: About the apparatus structure of respiratory apparatus for small animals. In: Arch. Tierernähr. 7 (1957), pp. 80-97.
Individual evidence
- ↑ R. Schiemann: For the gas-analytical investigation of respiratory gases. In: Arch. Tierernähr. 7, pp. 98-103 (1957).
- ↑ R. Schiemann, K. Nehring, L. Hoffmann, W. Jentsch, A. Chudy: Energetic food evaluation and energy norms. VEB Deutscher Landwirtschaftsverlag, Berlin 1971, DNB 456539212 .
- ^ E. Brouwer, 1965: Report of sub-committee on constants and factors. In: KL Blaxter (Ed.): Energy metabolism; proceedings of the 3rd symposium held at Troon. (= EAAP -Publ. 11). Academic Press, 1965, pp. 441-443.