Rieke metals
Rieke metals are a group of specially prepared, highly reactive metal powders . These are mostly in the form of a finely atomized suspension in tetrahydrofuran . As a result, on the one hand, no oxide layer forms on the surface and - with the same amount compared to a piece of metal - more surface is available. The manufacturing methods were developed by Reuben D. Rieke, who spent 40 years researching and manufacturing zinc-organic compounds and Grignard compounds . The active metals are freshly prepared in situ before the reaction by reducing the corresponding metal salts in tetrahydrofuran and are not separated from the salt formed (e.g. potassium chloride ). Usually potassium or lithium in connection with an electron carrier serve as reducing agents. They enable the preparation of organometallic compounds that are difficult to produce even under mild conditions.
Rieke magnesium
presentation
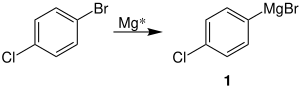
Rieke magnesium (Mg *) is produced from magnesium chloride by adding potassium and potassium iodide . The conditions are described in the literature.
Reactions
Rieke magnesium is particularly suitable for the production of Grignard compounds . An example of this is the reaction of halogenated benzene to form the organometallic compound 1 :
1 can then react further to alcohol 2 in a Grignard reaction, for example with the addition of benzaldehyde :
Rieke zinc
Manufacturing
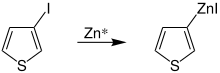
Rieke zinc (Zn *) can be obtained by reducing zinc chloride with potassium. More precise conditions are described in the literature.
Reactions
Rieke zinc forms organometallic compounds with various secondary or tertiary compounds, including halogenated heterocycles such as 2-iodothiophene, at room temperature :
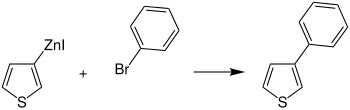
These organozinc compounds enable, for example, the substitution of organyl residues (e.g. aryl residues ) on the thiophene, which opens up a wide range of possibilities in the field of application of polythiophenes . An example is the conversion of an organozinc compound of thiophene to 2-phenylthiophene ( 1 ):
Rieke indium
Manufacturing
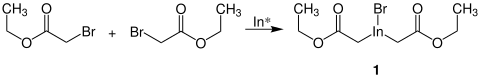
Rieke indium (In *) is represented using potassium as part of the reduction of indium chloride :
Reactions
Rieke indium is able to bind two brominated esters and thus form the organometallic compound 1 :
Such reagents are particularly suitable for a Reformatzki reaction . The reaction of a ketone with 1 results in β-hydroxy esters such as 2 :
Rieke calcium
Manufacturing
Rieke calcium (Ca *) can be produced by reducing calcium bromide . As a reducing agent acts lithium biphenyl - a lithium salt of biphenyl :
Reactions
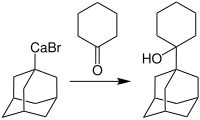
Rieke calcium is particularly suitable for the synthesis of organocalcium compounds . For example, brominated adamantane can react with Ca *:
Such reagents make it possible to synthesize important adamantane derivatives which are used in the treatment of dementia of the Alzheimer's type. The synthesis of 1- (1-adamantyl) cyclohexanol is given here as an application example:
Rieke copper
Manufacturing
Depending on the target product, Rieke copper (Cu *) is made from one of the three intercalation salts copper cyanide • lithium bromide , copper iodide • tributylphosphine or copper iodide • triphenylphosphine (CuI • PPh 3 ). Lithium naphthalene - a lithium salt of naphthalene - serves as a reducing agent:
Reactions
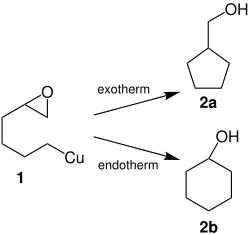
Rieke copper forms direct organocopper compounds with halogenated hydrocarbons, such as with 2- (4-bromobutyl) oxirane:
This organometallic compound ( 1 ) can then be converted to cyclopentylmethanol ( 2a ) or cyclohexanol ( 2b ) depending on the reaction conditions:
Rieke metals and polymers
Rieke metals can also be used for regioselective polymerizations. An example of this is the polymerization of iodinated benzene made possible by Rieke zinc (Zn *) via an organozinc compound to form polybenzene:
Another example that is highly relevant in practice is the regioselectively controlled polymerization of brominated thiophene to polythiophenes using Rieke zinc (Zn *) . This process is also known as the Rieke method:
Individual evidence
- ↑ a b Tse-Chong Wu, Heping Xiong, Reuben D. Rieke: Organocalcium Chemistry: Preparation and Reactions of Highly Reactive Calcium . In: The Journal of Organic Chemistry . 55, No. 17, August 1990, pp. 5045-5051. doi : 10.1021 / jo00304a016 .
- ^ Rieke Metals. Retrieved August 9, 2017 .
- ↑ a b Reuben D. Rieke, SE Bales, PM Hudnall, TP Burns, GS Poindexter: Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornane Acid . In: Organic Syntheses . 59, 1979, p. 85. doi : 10.15227 / orgsyn.059.0085 .
- ^ Reuben D. Rieke: Preparation of Organometallic Compounds from Highly Reactive Metal Powders . In: Science . 246, No. 4935, December 1989, pp. 1260-1264. doi : 10.1126 / science.246.4935.1260 .
- ↑ a b c d Reuben D. Rieke, Loretta I. Rieke, Tse-Chong Wu: Highly Reactive Calcium for the Preparation of Organocalcium Reagents: 1-Adamantyl Calcium Halides and Their Addition to Ketones: 1- (1-Adamantyl) cyclohexanol . In: Organic Syntheses . 72, 1995, p. 147. doi : 10.15227 / orgsyn.072.0147 .
- ↑ a b c d e f g h i j k l m n o Reuben D. Rieke, Mark V. Hanson: New Organometallic Reagents Using Highly Reactive Metals . In: Tetrahedron . 53, No. 6, 1997, pp. 1925-1956. doi : 10.1016 / S0040-4020 (96) 01097-6 .
- ↑ a b Jun-sik Lee, Raffet Velarde-Ortiz, Albert Guijarro, Joshua R. Wurst, Reuben D. Rieke: Low-Temperature Formation of Functionalized Grignard Reagents from Direct Oxidative Addition of Active Magnesium to Aryl Bromides . In: The Journal of Organic Chemistry . 65, 2000, pp. 5428-5430. doi : 10.1021 / jo000413i .