Polythiophenes

| Some thiophene derivatives (left) and derived polythiophenes (right) | |
| Monomer | polymer |
 |
 |
 |
 |
Polythiophenes are chemical compounds from the group of polymers . They differ in their properties not only in their chain length n , but also in the substituents R 1 and R 2 , which in the simplest case can be hydrogen, but also any complex alkyl radicals or alkoxy radicals . In 2000 the scientists Alan J. Heeger , Alan MacDiarmid and Hideki Shirakawa received the Nobel Prize in Chemistry for the discovery and development of conductive polymers .
properties

The polythiophenes have - regardless of the respective radical - a π-electron system delocalized across the chain . This results in the most important common property of the polythiophenes: electrical conductivity , which is why polythiophenes are also referred to as synthetic metals . The polythiophenes are also optically active. The color of the fluorescence varies depending on the applied voltage , solvent and temperature (see illustrations).
Regioregularity
| Head and tail position in the thiophene |
|---|
 Thiophene |
In the case of bonds between individual thiophene molecules (or their derivatives), there are three possibilities as to which atoms these bonds can be attached to. Each thiophene molecule is initially assigned a head and a tail , similar to the isoprene rule (see figure). Derived from this, there are three possible links between two monomers (head-tail = tail-head):
- Head - head
- Head - tail
- Tail - tail
Derived from these 3 connection possibilities between two monomers, there are 4 possibilities for a chain course (see adjacent figure):
- ( Head - tail ) - ( head - tail )
- ( Head - head ) - ( tail - head )
- ( Head - head ) - ( tail - tail )
- ( Tail - tail ) - ( head - tail )
The different chain courses can be differentiated by means of NMR spectroscopy and have an impact on the electrical conductivity of the polythiophene. The following applies:
The higher the regioregularity - i.e. the more ordered the chain course - the higher the conductivity of the polythiophene. A chain course with a 2: 1 ratio of head – tail to head – head bonds has almost three times the conductivity compared to a randomly arranged chain. Furthermore, the arrangement of the individual monomers also has an impact on the appearance of the respective polythiophene. Regioregular polyalkylthiophenes synthesized by the Rieke method form crystalline, flexible, bronze-colored and metallic shimmering films. In contrast, randomly arranged polyalkylthiophenes formed amorphous and orange-colored films.
Application examples
| Examples |
|---|
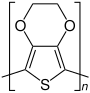
 PEDOT |
 "Fluorinated" polythiophene |
 Polythiophene wires |
Polythiophenes can be used in many ways. Research is constantly being carried out on variously substituted thiophenes in order to obtain certain properties.
PEDOT
The most relevant polythiophene in practice is poly-3,4-ethylenedioxythiophene (PEDOT). It is used in many ways in electrical engineering , for example in solar cell technology and as a component for capacitors . It is particularly practical for production that no regioregularity has to be observed.
Fluorinated polythiophene
A polythiophene that is relevant in research and practice is “fluorinated” polythiophene, which contains a perfluorinated carbon chain at the end of its remainder. To obtain this, thiophene can be carboxylated via a Grignard compound. Then - as explained in the synthesis section - either an electrochemical or chemical polymerization can take place. In addition to the electrical conductivity, the stability of fluorinated polythiophene against environmental influences is of particular interest, as it is water and oil repellent.
Polythiophene wires
At the beginning of the 21st century a method for the synthesis of small polythiophene wires was developed. The exact procedure is described in the literature. The polythiophene that the wires are made of is shown opposite. It contains a trimethylundecylammonium residue. The hexafluorophosphate anion is used as the counterion .
Manufacturing
Possible syntheses of a polythiophene can be done in different ways. It can first be divided into electrochemical and chemical synthesis processes. Both electrochemical and chemical synthesis each have their advantages and disadvantages for differently substituted thiophenes.
Electrochemical Synthesis
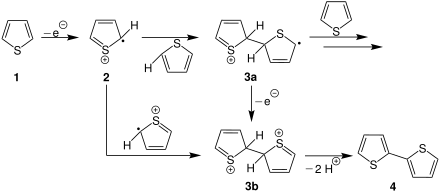
Electrochemical synthesis is one way of synthesizing polythiophenes. The principle is shown schematically on the right using the example of the reaction of the unsubstituted thiophene ( 1 ) to form the dithiophene ( 4 ). In the first step, a thiophene radical cation 2 is generated by oxidation . Two different courses of reaction are conceivable:
The first possibility is described in the course above. It consists in the fact that 2 attacks another thiophene molecule and the intermediate stage 3a is formed. This step can be repeated as often as required, which means that a polythiophene chain of any length can be created. Otherwise, the dication 3b can arise from intermediate 3a through oxidation .
The second conceivable course of the reaction starting from 2 is a termination reaction (bottom). This forms the dication 3b . 3b then dissociates to form the dithiophene ( 4 ).
Chemical synthesis
Chemical synthesis is particularly relevant for regioregular polythiophene chains. Frequently, a strong regioregularity of the chain course can be generated through regioselective partial reactions. There are essentially three established processes for chemical synthesis:
1. Rieke method
The Rieke method is based on the use of Rieke zinc (Zn *) and 2,5-dibromothiophene ( 1 , R = alkyl radical ):
This particularly reactive zinc is added at very low temperatures and leads to the regioselective formation of the organic zinc compound 2 . 2 then reacts as a monomer with the addition of a nickel chloride complex with dppe and forms the highly regioregular (head-to-tail) polyalkylthiophene 3 .
2. McCullough method
The McCullough method follows a similar principle as the Rieke method:
First, the alkylthiophene ( 1 , R = alkyl radical) is brominated by the known brominating agent N- bromo succinimide (NBS), so that 2-bromothiophene 2 is formed. Then 2 is first reacted with lithium diisopropylamide (LDA). The resulting lithium-organic compound is with magnesium bromide (MgBr 2 ) is a transmetalation and is finally the by the addition of nickel chloride-dppp complex ([Ni (dppp) Cl 2 ]) in a Kumada coupling the Alkylpolythiophen 3 obtained which by the regioselectivity the reaction shows a high regioregularity (head-tail).
3. Oxidation by iron (III) chloride / iron (III) sulfate
In practice, the synthesis of certain polythiophenes - such as poly-3,4-ethylenedioxythiophene - through the use of iron (III) salts plays an important role. The underlying mechanism has been widely discussed historically since the 1980s . Three possible courses of reaction are conceivable. Schematically, all reactions consist of the elements oxidation , deprotonation and an electrophilic or radical attack of the eponymous intermediate. Three possible intermediate stages are suggested that determine the respective course of the reaction - radical ( 3a , top), carbocation ( 2b , center), radical cation ( 2c , bottom). The graphic shows an example of the formation of the trimer from a dimer .
Individual evidence
- ↑ Giovanna Barbarella, Alessandro Bongini, Massimo Zambianchi: Regiochemistry and Conformation of Poly (3-hexylthiophene) via the Synthesis and the Spectroscopic Characterization of the Model Configurational Triads . In: Macromolecules . 27, No. 11, 1994, pp. 3039-3045. doi : 10.1021 / ma00089a022 .
- ^ GA Diaz-Quijada: Regiochemical Analysis of Water Soluble Conductive Polymers: Sodium Poly (ω- (3-thienyl) alkanesulfonates) . In: Macromolecules . 29, No. 16, 1996, pp. 5416-5421. doi : 10.1021 / ma960126 .
- ↑ RL Elsenbaumer, K.-Y. Jen, GG Miller, H. Eckhardt, LW Shacklette, R. Jow: Poly (alkyl thiophenes) and Poly (substituted heteroaromatic vinylenes): Versatile, Highly Conductive, Processible Polymers with Tunable Properties . In Electronic Properties of Conjugated Polymers (Eds: Kuzmany, H .; Mehring, M .; Roth, S.), Springer, Berlin, 1987, ISBN 0-387-18582-8
- ↑ a b c Tian-An Chen, Xiaoming Wu, Reuben D. Rieke: Regiocontrolled Synthesis of Poly (3-alkylthiophenes) Mediated by Rieke Zinc: Their Characterization and Solid-State Properties . In: Journal of the American Chemical Society . 117, 1995, p. 233. doi : 10.1021 / ja00106a027 .
- ↑ St. Kirchmeyer, D. Gaiser, HC Starck GmbH & Co KG: Electronic components: extremely flat and flexible
- ↑ Andreas Elschner, Stephan Kirchmeyer, Wilfried Lovenich, Udo Merker, Knud Reuter: PEDOT: Principles and Applications of an Intrinsically Conductive Polymer . CRC Press, 2010, ISBN 978-1-4200-6912-9 .
- ^ A b Mael Nicolas, Frédéric Guittard, Serge Géribaldi: Synthesis of Stable Super Water- and Oil-Repellent Polythiophene Films. In: Angewandte Chemie . 2006, pp. 2309-2312. doi: 10.1002 / anie.200503892 .
- ↑ Guangtao Li, Sheshanath Bhosale, Tianyu Wang, Yang Zhang, Hesun Zhu, Jürgen-Hinrich Fuhrhop: Synthesis of molecular submicrometer-long polythiophene wires on the gram scale in mesoporous silicate matrices . In: Angewandte Chemie . 115, 2003, pp. 3948-3951. doi : 10.1002 / anie.200351158 .
- ^ J Roncali, R. Garreau, A. Yassar, P. Marque, F. Garnier, M. Lemaire: Effects of steric factors on the electrosynthesis and properties of conducting poly (3-alkylthiophenes) . In: The Journal of Physical Chemistry . 91, No. 27, December 1987, pp. 6706-6714. doi : 10.1021 / j100311a030 .
- ^ Richard D. McCullough: The Chemistry of Conducting Polythiophenes . In: Advanced Materials . 10, No. 2, January 1998, pp. 93-116. doi : 10.1002 / (SICI) 1521-4095 (199801) 10: 2 <93 :: AID-ADMA93> 3.0.CO; 2-F .
- ↑ VM Niemi, P. Knuuttila, J.-E. Österholm: Polymerization of 3-alkylthiophenes with FeCl3 . In: polymer . 33, No. 7, 1992, pp. 1559-1562. doi : 10.1016 / 0032-3861 (92) 90138-M .
- ↑ MR Andersson, D. Selse, M. Berggren, H. Jaervinen, T. Hjertberg, O. Inganaes, J. -E. Oesterholm: Regioselective polymerization of 3- (4-octylphenyl) thiophenes with FeCl3 . In: Macromolecules . 27, No. 22, October 1994, pp. 6503-6505. doi : 10.1021 / ma00100a039 .
- ↑ Giovanna Barbarella, Massimo Zambianchi, Rosanna Di Toro, Martino Colonna Jr., Dario Iarossi, Francesca Goldoni, Alessandro Bongini: Regioselective Oligomerization of 3- (Alkylsulfanyl) thiophenes with Ferric Chloride . In: The Journal of Organic Chemistry . 61, No. 23, November 1996, pp. 8285-8292. doi : 10.1021 / jo960982j .