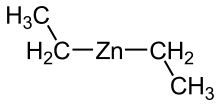Organic zinc compounds
Organic zinc compounds are chemical compounds of zinc with organic residues. Like many other organometallic compounds, they are reactive and react quickly with air or water. Organic zinc compounds play a role as reagents in organic reactions, including the Reformatzki reaction and the Negishi coupling .
history
The organozinc compounds dimethylzinc and diethylzinc were the first known organometallic compounds with a σ-bond between metal and carbon . They were discovered by chance in 1849 by Edward Frankland , who was trying to extract radicals from alkyl iodides and zinc . Before this discovery, only organic compounds of the semimetal arsenic and Zeise's salt were known as the first complex with organic ligands .
Extraction and presentation
Organic zinc compounds can be represented in several ways. One possibility is the direct synthesis from metallic zinc and alkyl iodides via an unstable alkyl zinc iodide intermediate.
Also transmetalation from mercury-organic compounds , as well as metathesis reactions with zinc halides and organic lithium - or aluminum compounds are possible to represent organic zinc compounds.
properties
In contrast to organomagnesium or beryllium compounds, organic zinc compounds have a monomeric structure. The molecules are linear; bridges in which two zinc centers are linked by a common organic group are not stable. Such 2-electron-3-center bonds are possible via hydrogen atoms .
Due to the similar structure due to the closed d-shell, organic zinc compounds resemble Grignard compounds in their chemical behavior . However, due to the stronger covalent bond and lower Lewis acidity, they are less reactive than these and therefore react more specifically than Grignard compounds.
use
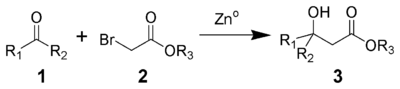
In the Reformatzki reaction , an α- halogenated carboxylic acid ester and zinc initially form an organic zinc compound which, as a nucleophile , can then attack a ketone or an aldehyde . In contrast to Grignard reagents, which can enter into comparable reactions, the Zink-Reformatski reagent does not react with carboxylic acid esters.
Another reaction in which organic zinc compounds are the starting materials is the Negishi coupling . In this reaction, organozinc compounds and aryl halides react with one another under palladium or nickel catalysis to form a CC bond.
Zinc carbenes , in which there is a carbon-zinc double bond, play a role in the Simmons-Smith reaction . First of all, diiodomethane and zinc are used to generate the carbene, which reacts with alkenes to form cyclopropane derivatives .
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1496-1497.
- Christoph Elschenbroich : Organometallic chemistry. 6th edition, Teubner Wiesbaden, 2008, ISBN 978-3-8351-0167-8 , pp. 72-76.