Rifaximin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
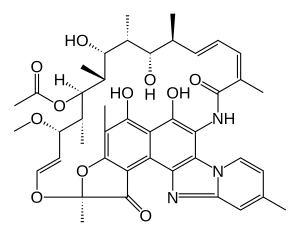
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Rifaximin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 43 H 51 N 3 O 11 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Protein synthesis inhibitor |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 785.88 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Rifaximin , a semi-synthetic derivative of rifamycin , is a bactericidal broad spectrum oral antibiotic .
Due to the pyridoimidazole structure, rifaximin is practically not absorbed (<1%) and is only effective in the intestinal lumen , which distinguishes it from systemic antibiotics .
The broad spectrum of germs, the lack of systemic side effects and interactions as well as the practically unobserved development of resistance make rifaximin a therapeutic option for intestinal bacterial infections .
application areas
Approved indications in Germany
In Germany, rifaximin is approved for the treatment of travelers' diarrhea in adults caused by non-invasive enteropathogenic bacteria (such as Escherichia coli ) , which is defined as diarrhea in travelers acquired in a Mediterranean, subtropical or tropical country. Since 2013, Rifaximin has also been approved in Germany for the treatment and prophylaxis of hepatic encephalopathy .
The approval is based, among other things, on a randomized double- blind study in which rifaximin was tested against ciprofloxacin . 187 patients with diarrhea received either 400 mg rifaximin or 500 mg ciprofloxacin twice daily for three days. The number of sick patients decreased in a comparable way in both groups. Both antibiotics shortened the duration of diarrhea by around a day.
Internationally approved indications
The properties of rifaximin have been described in the medical literature since 1983.
To date, the effectiveness and tolerability have been proven in numerous clinical studies, which have led to further approvals internationally. Rifaximin is used in other countries, such as B. Austria, approved for the treatment of the following diseases:
- Bacterial overgrowth syndrome (overgrowth of the small intestine ) *
- Clostridium difficile * pseudomembranous colitis
- Hepatic encephalopathy
- Diverticular disease *
- Preoperative bowel decontamination *
(* these indications are not yet approved in Germany)
Rifaximin status worldwide
The drug has been available in Germany since 2008. The active ingredient is sold in 17 countries worldwide.
Pharmacological information
After oral administration, less than 1% of rifaximin is absorbed via the gastrointestinal tract. This also applies to patients whose mucosa (intestinal lining) is damaged by inflammation and ulceration. Very high concentrations of up to 8000 μg / g are reached in the intestine after oral administration of rifaximin. This means that the concentration of the active substance is significantly higher than the minimum inhibitory concentration (MIC) of the bacterial pathogen. Rifaximin is excreted almost entirely unchanged in the stool.
effect
In two small studies, rifaximin halved the time to the last unformed stool compared to placebo. The little-tested agent has not been adequately tested for children and the elderly.
Antibiotic therapy is controversial because it promotes the development of resistance. In addition, potential disruptive effects such as allergic reactions must be considered.
The Medicinal Telegram sees no indication for Rifaximin in traveller's diarrhea.
Mechanism of action
Like other antibiotics of the rifamycin group, rifaximin binds irreversibly to the beta subunit of the prokaryotic DNA-dependent RNA polymerase. In this way it blocks the binding of the enzyme to the DNA and thus the initiation of chain formation. By suppressing RNA transcription, protein synthesis is ultimately inhibited. The effect of rifaximin is bactericidal.
Spectrum of activity
The active ingredient rifaximin has a broad spectrum of antimicrobial activity against most gram-positive and negative, aerobic and anaerobic bacteria and thus covers most of the bacteria that cause intestinal infections.
| Gram-positive pathogens | Gram-negative pathogens | ||
|---|---|---|---|
| Aerobes | Anaerobes | Aerobes | Anaerobes |
|
|
|
|
|
Microaerophiles
|
|||
Tab. 1: Selection of rifaximin-sensitive pathogens
Adverse effects (side effects)
Rifaximin has a favorable tolerability profile, which can be derived from its minimal systemic availability. In studies, the frequency is at the placebo level.
| Rifaximin (600 mg / d) n = 320 |
Placebo n = 228 |
|
|---|---|---|
| (% Patients) | ||
| flatulence | 9.7 | 19.3 |
| Abdominal pain | 5.9 | 9.2 |
| Nausea | 4.7 | 8.3 |
| Rectal tenesmus | 4.1 | 6.1 |
| Urge to defecate | 3.8 | 6.6 |
| Constipation | 3.4 | 2.6 |
| Headache | 5.3 | 5.7 |
| fever | 3.1 | 4.4 |
Table 2: Adverse events reported in the registration studies of Rifaximin
Drug interactions
There are no indications of interactions between rifaximin and other medicinal products.
Since the absorption of orally administered rifaximin via the gastrointestinal tract is negligible (<1%), no systemic interactions with other medicinal products are to be assumed.
Contraindications (contraindications)
Rifaximin should not be used in patients with clinical signs of invasive enteritis, e.g. B. with fever or bloody stool. If you are hypersensitive to the active ingredient, to other rifamycin derivatives or to any of the other ingredients, Rifaximin must not be used. There is no experience with the treatment of children.
Others
- Rifaximin has been available in Italy since 1987 and in 2009 it was awarded the HG Creutzfeldt Institute's innovation prize in Germany .
- In 2005, Rifaximin received orphan drug status from the American Food and Drug Administration (FDA) for hepatic encephalopathy (HE) .
- The United Nations Medical Services Department recommended that members of the organization take the German preparation with them in the first-aid kit.
Trade names
Monopreparations Colidimin (A), Xifaxan (CH, D), Rifacol (I)
Individual evidence
- ↑ a b c Rifaximin data sheet at Sigma-Aldrich , accessed on December 1, 2019 ( PDF ).
- ^ A b J.J. Descombe, D. Dubourg, M. Picard, E. Palazzini: Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. In: Int J Clin Pharmacol Res. 14 (2), 1994, pp. 51-56.
- ↑ a b W. W. Hoover, EH Gerlach, DJ Hoban, GM Eliopoulos, MA Pfaller, RN Jones: Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. In: Diagn Microbiol Infect Dis. 16, 1993, pp. 111-118.
- ^ A b c Veteran Health Administration. VHA Pharmacy Benefits Management Strategic Healthcare Group and Medical Advisory Panel. National PBM Drug Monograph. Rifaximin (Xifaxan ™) ( Memento from December 15, 2006 on the Internet Archive ) (2004).
- ↑ a b c L. L. Brunton, JS Lazo, Parker KL (eds): Goodman & Gilman's The pharmacological basis of therapeutics. 11th edition. New York et al. 2006.
- ↑ C. De Leo, C. Eftimiadi, GC Schito: Rapid disappearance from the intestinal tract of bacteria resistant to rifaximin. In: Drugs Exptl Clin Res. 12, 1986, pp. 979-981.
- ↑ HL DuPont, ZD Jiang: Influence of rifaximin treatment on the susceptibility of intestinal Gram-negative flora and enterococci. In: Clin Micro Infect Dis. 10 (11), 2004, pp. 1009-1011.
- ↑ HL DuPont, ZD Jiang, CD Ericsson, JA Adachi, JJ Mathewson, MW DuPont, E. Palazzini, LM Riopel, D. Ashley, F. Martinez-Sandoval: Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double - blind clinical trial. In: Clin Infect Dis . 33, 2001, pp. 1807-1815.
- ^ F. Castelli, N. Saleri, LR Tomasoni, G. Carosi: Prevention and treatment of traveler's diarrhea. Focus on antimicrobial agents. In: Digestion. 73 (suppl 1), 2006, pp. 109-168.
- ↑ HL DuPont, ZD Jiang, CD Ericsson, JA Adachi, JJ Mathewson, MW DuPont, E. Palazzini, LM Riopel, D. Ashley, F. Martinez-Sandoval: Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double - blind clinical trial. In: Clin Infect Dis. 33, 2001, pp. 1807-1815. PMID 11692292 .
- ↑ a b P. Gionchetti, F. Rizzello, A. Venturi, F. Ugolini, M. Rossi, P. Brigidi, R. Johansson, A. Ferrieri, G. Poggioli, M. Campieri: Review antibiotic treatment in inflammatory bowel disease: rifaximin, a new possible approach. In: Eur Rev Med Pharmacol Sci . 3 (1), 1999, pp. 27-30. PMID 10710827 .
- ↑ ZD Jiang, S. Ke, E. Palazzini, L. Riopel, H. Dupont: In vitro activity and fecal concentration of rifaximin after oral administration. In: Antimicrob Agents Chemother . 44 (8), 2000, pp. 2205-2206. PMID 10898704 ; PMC 90042 (free full text).
- ^ R. Steffen, DA Sack, L. Riopel, ZD Jiang, M. Stürchler, CD Ericsson, B. Lowe, P. Waiyaki, M. White, HL DuPont: Therapy of travelers' diarrhea with rifaximin on various continents. In: American Journal of Gastroenterology . Volume 98, Number 5, May 2003, pp. 1073-1078, doi: 10.1111 / j.1572-0241.2003.07283.x . PMID 12809830 .
- ↑ DN Taylor, AL Bourgeois, CD Ericsson, R. Steffen, ZD Jiang, J. Halpern, R. Haake, HL Dupont: A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers 'diarrhea. In: The American journal of tropical medicine and hygiene. Volume 74, Number 6, June 2006, pp. 1060-1066. PMID 16760520 .
- ↑ Arznei-Telegramm at 39, 2008, pp. 121–3.
- ↑ a b K. U. Petersen: Rifaximin: A locally effective antibiotic for the gastrointestinal tract. Premium Selection 2008.
- ↑ RIFAXIMIN (XIFAXAN) IN CASE OF TRAVEL DIAGNOSIS? In: Medicinal Telegram. December 2008.
- ↑ Sabine Stürmer: Innovation award for intestinal antibiosis. In: Doctors newspaper. April 22, 2009.
- ↑ US Food and Drug Administration: List of orphan products designation and approvals (PDF; 6 MB) as of October 3, 2007.
- ↑ Press release Salix Pharmaceuticals, Inc., August 4, 2008.