Semustin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Structure without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Semustin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 18 ClN 3 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 247.72 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
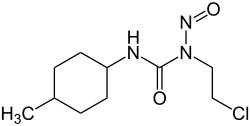
Semustine (synonym: Me thyl- C hlorethyl- C yclohexyl- N itroso- U rea = MeCCNU ), chemically 1- (2-chloroethyl) -3- (4-methylcyclohexyl) -1-nitrosourea , which is non-proprietary name for a cytostatic agent from the group of nitrosoureas . It is the 4-methyl derivative of lomustine .
Chemical properties
Stereochemistry
Semustine is a compound of which two isomers , so-called cis / trans isomers, exist due to the different substitution options on the cyclohexane ring . These isomers can differ in certain physical properties and physiological effects. The individual compounds can be isolated in a targeted manner by means of suitable synthesis strategies or separation processes.
pharmacology
Mechanism of action
Semustine changed as all nitrosoureas the genetic material in the cell nuclei . As a result, the affected cells can no longer divide and die after a while. The genome damage mainly affects rapidly dividing cells - especially cancer cells. Semustine is able to cross the blood-brain barrier . This is beneficial in the treatment of melanoma with CNS metastatisation.
Side effects
Semustine is myelotoxic , which means it damages the bone marrow . This damage has a negative effect on blood formation, especially platelets and leukocytes . The main side effects are nausea and hair loss .
Like all nitrosoureas, Semustine is itself carcinogenic , i.e. carcinogenic. In animal experiments it increases the total tumor rate in rats after intraperitoneal administration and the rate of leukemia and malignant lymphomas in female mice . It has been classified as carcinogenic for humans since the early 1990s. The risk of developing leukemia increases significantly after treatment with Semustin.
Individual evidence
- ↑ a b Datasheet Semustine at Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ Elizabeth K. Weisburger : Bioassay program for carcinogenic hazards of cancer chemotherapeutic agents. In: Cancer 40, 1977, S, 1935-1949, PMID 907995 .
- ↑ H. Tinwell and J. Ashby: Activity of the human carcinogen MeCCNU in the mouse bone marrow micronucleus assay. In: Environ Mol Mutagen 17, 1991, pp. 152-154, PMID 2022193 .
- ↑ JD Boice et al.: Leukemia and preleukemia after adjuvant treatment of gastrointestinal cancer with semustine (methyl-CCNU). In: NEJM 309, 1983, pp. 1079-1084, PMID 6353233 .
literature
- 13th Report on Carcinogens (RoC): Nitrosourea Chemotherapeutic Agents ( 1- (2-Chloroethyl) -3- (4-Methylcyclohexyl) -1-Nitrosourea ) (PDF file; 233 kB)
- Collective of authors: Radiation therapy and fluorouracil with or without semustine for the treatment of patients with surgical adjuvant adenocarcinoma of the rectum. Gastrointestinal Tumor Study Group. In: Journal of Clinical Oncology . 10, 1992, pp. 549-557. PMID 1548520
- National Toxicology Program: 1- (2-Chloroethyl) -3- (4-methylcyclohexyl) -1-nitrosourea (MeCCNU). In: Rep Carcinog 10, 2002, pp. 53-54. PMID 15320318
- RS Witte et al .: PALA versus streptozotocin, doxorubicin, and MeCCNU in the treatment of patients with advanced pancreatic carcinoma. In: Invest New Drugs 16, 1998-1999, pp. 315-318. PMID 10426663
- JL Clark et al: Adjuvant 5-FU and MeCCNU improves survival following curative gastrectomy for adenocarcinoma. In: Am Surg 56, 1990. pp. 423-427. PMID 2368986
- M. Willem: Adjuvant therapy for rectal cancer: is 5-FU effective without MeCCNU. In: Oncology (New York) 3, 1989, p. 11. PMID 2641312
- RC Vyas et al: Genotoxic effects of MeCCNU on human peripheral blood lymphocytes. In: Toxicology Letters 44, 1988, pp. 153-159. PMID 3188073