Sesamex
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
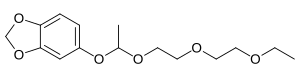
|
|||||||||||||
| Structural formula without stereochemistry | |||||||||||||
| General | |||||||||||||
| Surname | Sesamex | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 15 H 22 O 6 | ||||||||||||
| Brief description |
straw yellow liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 298.33 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| solubility |
very sparingly soluble in water: 0.155 g l −1 (20 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Sesamex ( Sesoxan ) acts as a synergist and strengthens the insecticidal effect of other insecticides such as pyrethrins , pyrethroids or carbamates . Taken by itself, it has no effect on insects. It was developed in 1950 by Morten Beroza from the Agricultural Research Service based on sesamol .
Admission
Sesamex is not approved as a crop protection agent in the European Union or Switzerland .
Individual evidence
- ↑ NP Cheremisinoff, JA King: Toxic Properties of Pesticides . Marcel Dekker Inc, 1994 ( limited preview in Google Book Search).
- ↑ Entry on Sesamex in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on May 6, 2014.
- ↑ Entry on 5- (3,6,9-Trioxa-2-undecyloxy) benzo (d) -1,3-dioxolane in the GESTIS substance database of the IFA , accessed on July 10, 2016(JavaScript required) .
- ↑ Entry on 5- (3,6,9-trioxa-2-undecyloxy) benzo (d) -1,3-dioxolane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Distributors can expand the harmonized classification and labeling .
- ↑ Patent US2832792 : 3,4-methylenedioxyphenyl acetals as synergists for pyrethrins. Registered on June 15, 1955 , published April 29, 1958 , inventor: Morten Beroza.
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national directory of plant protection products in Switzerland ; Retrieved June 25, 2016.
