Asymmetric dihydroxylation
The asymmetric dihydroxylation is one of Barry Sharpless developed catalytic asymmetric oxidation of an olefin to a cis-vicinal diol . The high enantioselectivity of the reaction comes about through the use of a chiral ligand .
Reagents
The reaction is carried out with a catalytic amount of osmium (VIII) oxide , which is usually added as an Os (VI) salt and in the course of the reaction from a cooxidant - K 3 Fe (CN) 6 or NMO - to Os (VIII) -Species is oxidized.
Dihydroquinine or dihydroquinidine derivatives are often added as chiral ligands, which are commercially available as AD mix . In addition to K 2 OsO 2 (OH) 4 , K 3 Fe (CN) 6 and K 2 CO 3, AD-Mix α contains the ligand (DHQ) 2 PHAL in catalytic amounts, while AD-Mix β as ligands (DHQD) 2 PHAL contains. A two-phase mixture - for example tert- butanol and water - is used as a solvent in order to prevent premature reoxidation of the cyclic osmate and the associated loss of enantioselectivity. In addition, methanesulfonamide is often used, which accelerates hydrolysis and acts as a phase transfer catalyst.
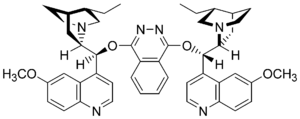
The osmium (VIII) oxide is bound via a free electron pair in the sp 3 atom orbital of the nitrogen of a dihydroquinidine (see graphic), which accelerates the ligand and allows the entire reaction to proceed via the reaction path of the chiral complex . This in turn results in the high enantioselectivity. Due to the conformation of the ligand and the osmium (VIII) oxide bound to it, a “chiral pocket” is formed, similar to the active center of an enzyme, in which the olefin only orientates itself in a certain conformation.
Reaction mechanism
From the olefin and the osmium (VIII) oxide ligand complex, a [3 + 2] cycloaddition results in a five-membered cyclic intermediate that hydrolyzes and releases the diol . In addition, the reduced osmate is produced, which is reoxidized by the cooxidant added in excess of stoichiometry.
Individual evidence
- ↑ Makoto Minato, Keiji Yamamoto and Jiro Tsuji : Osmium Tetraoxide Catalyzed Vicinal Hydroxylation of Higher Olefins by Using Hexacyanoferrate (III) Ion as a Cooxidant. In: The Journal of Organic Chemistry . Vol. 55, No. 2, 1990, pp. 766-768, doi: 10.1021 / jo00289a066 .
- ↑ Philippe Dupau, Robert Epple, Allen A. Thomas, Valery V. Fokin and K. Barry Sharpless: osmium-Catalyzed Dihydroxylation of olefin in Acidic Media: Old Process, New Tricks. In: Advanced Synthesis & Catalysis . Volume 344, No. 3-4, June 2002, pp. 421-433, ISSN 1615-4150 , doi : 10.1002 / 1615-4169 (200206) 344: 3/4 <421 :: AID-ADSC421> 3.0.CO; 2-F .
- ↑ Mikko H. Junttila and Osmo OE Hormi: Methanesulfonamide: A Cosolvent and a General Acid Catalyst in Sharpless Asymmetric Dihydroxylations. In: The Journal of Organic Chemistry. Volume 74, No. 8, 2009, pp. 3038-3047, doi: 10.1021 / jo8026998 .
- ^ EJ Corey , Mark C. Noe and Sepehr Sarshar: X-Ray Crystallographic Studies Provide Additional Evidence that an Enzyme-Like Binding Pocket is Crucial to the Enantioselective Dihydroxylation of Olefins by OsO 4 -bis -cinchona Alkaloid Complexes. In: Tetrahedron Letters . Volume 35, No. 18, May 2, 1994, pp. 2861-2864, doi: 10.1016 / S0040-4039 (00) 76644-5 .
- ↑ Hartmuth C. Kolb, Pher G. Andersson and K. Barry Sharpless: Toward an Understanding of the High Enantioselectivity in the osmium-Catalyzed Asymmetric Dihydroxylation (AD). 1. Kinetics. In: Journal of the American Chemical Society. Volume 116, No. 4, 1994, pp. 1278-1291, doi: 10.1021 / ja00083a014 .
literature
- Hartmuth C. Kolb , Michael S. Van Nieuwenhze and K. Barry Sharpless: Catalytic Asymmetric Dihydroxylation. In: Chemical Reviews . Volume 94, No. 8, 1994, pp. 2483-2547, doi: 10.1021 / cr00032a009 .
- Reinhard Brückner : reaction mechanisms. Organic reactions, stereochemistry, modern synthetic methods. 2nd edition, Spektrum Akademischer Verlag, Heidelberg and Berlin 2003, ISBN 3-8274-1189-0 , pp. 753-758.
- Jie Jack Li and EJ Corey : Name Reactions of Functional Group Transformations. Wiley Interscience, New York 2007, ISBN 978-0-471-74868-7 , pp. 67-83.