tert -butanol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | tert -butanol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 10 O | ||||||||||||||||||
| Brief description |
colorless solid with a camphor-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 74.12 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.79 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
26 ° C |
||||||||||||||||||
| boiling point |
83 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
completely miscible with water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 20 ml m −3 or 60 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
tert -butanol (according to IUPAC nomenclature: 2-methyl propan-2-ol , also known as tert -butyl alcohol known) is an organic chemical compound and einfachster representatives of the material group of the tertiary alcohols .
Extraction and presentation
Tert-butanol is produced on an industrial scale by acid-catalyzed hydration of isobutene at temperatures of 30–120 ° C. and pressures of 5–12 bar. Acid ion exchange resins are predominantly used as the catalyst .
This reaction almost exclusively supplies the tert-butanol and not the isomeric isobutanol , since - according to Markovnikov's rule - the thermodynamically most stable carbenium ion (here: tert-butyl cation) is always formed.
properties
Physical Properties
2-Methyl-2-propanol is a solid, colorless substance that melts at just above room temperature and has a characteristic smell of camphor . As a solid, the compound occurs in three polymorphic crystal forms. At a temperature of 13 ° C, crystal form II changes into crystal form I with a conversion enthalpy of 0.828 kJ · mol −1 . This then melts at 26 ° C with a melting enthalpy of 6.703 kJ mol −1 . Another metastable crystal form III shows a conversion to crystal form I at 21 ° C with a conversion enthalpy of 0.490 kJ mol −1 .
The compound forms azeotropically boiling mixtures with a number of other solvents .
Azeotropes with various solvents solvent water n -hexane n -heptane Cyclohexane benzene Tert-butanol content in mol% 64.6 24.7 68.8 40.0 37.8 boiling point in ° C 79 64 78 71 74 Heat of evaporation in kJ mol −1 37.92 33.28 38.40 35.52 35.53
Thermodynamic properties
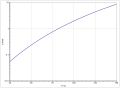
According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.49774, B = 1174.869 and C = −93 , 92 in the temperature range from 312.66 to 355.56 K. The temperature dependence of the enthalpy of vaporization can be calculated according to the equation Δ V H 0 = A · e (−αT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature) with A = 69.085 kJ / mol, α = −0.3583, β = 0.678 and T c = 506.2 K in the temperature range between 298 K and 385 K. .
Compilation of the most important thermodynamic properties property Type Value [unit] Remarks Standard enthalpy of formation Δ f H 0 liquid
Δ f H 0 gas−359.2 kJ mol −1
−312.6 kJ mol −1Standard entropy S 0 liquid
S 0 solid189.5 J mol −1 K −1
170.87 J mol −1 K −1as a liquid
as a solidEnthalpy of combustion Δ c H 0 liquid −2644.0 kJ mol −1 Heat capacity c p 215.37 J mol −1 K −1 (25 ° C)
2.91 J g −1 K −1 (25 ° C)
113.63 J mol −1 K −1 (25 ° C )
1.53 J g −1 K −1 (25 ° C)as a liquid
as a gasCritical temperature T c 506.2 K Critical pressure p c 39.7 bar Enthalpy of fusion Δ f H 6.7 kJ mol −1 at the melting point Enthalpy of evaporation Δ V H 0
Δ V H46.74 kJ mol −1
39.07 kJ mol −1
at normal pressure boiling point
Vapor pressure function of tert-butanol
Temperature dependence of the heat of evaporation of tert-butanol
Safety-related parameters
2-methyl-2-propanol is a highly flammable solid. The compound has a flash point of 11 ° C, which means that below the melting point above solid 2-methyl-2-propanol, flammable vapor-air mixtures can form. The explosion range is between 1.4% by volume (43 g / m 3 ) as the lower explosion limit (LEL) and 10.9% by volume (250 g / m 3 ) as the upper explosion limit (UEL). Correlating the explosion limits with the vapor pressure function results in a lower explosion point of 9 ° C. The limit gap width was determined to be 1.01 mm. This results in an assignment to explosion group IIA. The ignition temperature is 470 ° C. The substance therefore falls into temperature class T1.
Chemical properties
Like all aliphatic alcohols, the hydroxyl group of 2-methyl-2-propanol can be deprotonated and the tert- butylate anion is obtained . A well-known salt that is often used in synthetic organic chemistry is potassium tert -butanolate . B. is accessible by reaction of 2-methyl-2-propanol with elemental potassium.
This strongly basic salt is used as a sterically demanding and therefore only weakly nucleophilic base, for example in deprotonations in which the base must not attack nucleophilically. Often 2-methyl-2-propanol is also used as a solvent.
Strong protic acids ( hydrochloric acid , sulfuric acid, phosphoric acid etc.) protonate 2-methyl-2-propanol at the oxygen atom and the tert- butyl cation is formed with the elimination of water, which is stabilized by the hyperconjugation effect of the three methyl groups. In the presence of good nucleophiles, the reaction proceeds in the sense of a nucleophilic substitution (S N 1) . For example, 2-chloro-2-methylpropane ( tert-butyl chloride) is formed very easily from 2-methyl-2-propanol and concentrated hydrochloric acid. If no suitable nucleophiles are present (e.g. when using sulfuric acid or phosphoric acid), the reaction proceeds as an elimination (E1) to 2-methylpropene (isobutene). This is the reverse of the production of 2-methyl-2-propanol.
use
tert -Butanol is used as a fuel additive to prevent carburetor icing or as an anti- knock agent . The alcohol also serves as a starting material for the synthesis of tert-butyl esters and tert-butyl phenols, which in turn are used as antioxidants . It is also used as a solvent in the cryoscopic determination of molar mass and in the gas chromatographic determination of the blood alcohol concentration ( headspace GC ) as an internal standard. Furthermore, tert- butanol is used as an additional denaturing agent for drinking alcohol (ethanol).
In synthetic chemistry it is u. a. also used to dispose of residues of the alkali metals potassium and sodium , as it reacts in a controllable manner to form tert- butanolates.
Safety instructions / risk assessment
2-methyl-2-propanol can cause irritation on skin and eye contact. When inhaled, it continues to cause coughing, which is how it is also reabsorbed by the body . If swallowed, nausea and vomiting may occur. After absorption, drowsiness, dizziness, respiratory paralysis, drop in blood pressure and cardiovascular disorders may occur. 2-methyl-2-propanol is slightly hazardous to water ( water hazard class 1).
In 2013, tert -butanol was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes for the uptake of tert- butanol were concerns about consumer use , high (aggregated) tonnage, high risk characterization ratio (RCR) and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances. The re-evaluation has been running since 2013 and is carried out by the UK . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry on 2-methyl-2-propanol in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ Entry on 2-methylpropan-2-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 75-65-0 or tert-butanol ), accessed on November 2, 2015.
- ↑ Andreas Beckmann, Wilfried Büschken, Silvia Santiago Fernandez, Alfred Kaizik, Franz Nierlich, Dieter Reusch, Bernhard Scholz: Process for the production of tert-butanol. In: Google Patents. EVONIK OXENO GMBH, December 19, 2002, accessed on December 9, 2018 .
- ↑ a b c d e F. L. Oetting: The heat capacity and entropy of 2-methyl-2-propanol from 15 to 330 K. In: J. Phys. Chem. 67, 1963, pp. 2757-2761, doi: 10.1021 / j100806a059 .
- ↑ a b Y. Demirel: Estimation of the entropy of vaporization at the normal boiling point for azeotropic mixtures containing water, alcohol or acetic acid. In: Thermochim. Acta . 339, 1999, pp. 79-85, doi: 10.1016 / S0040-6031 (99) 00211-7 .
- ^ I. Brown, W. Fock, F. Smith: The Thermodynamic Properties of Solutions of Normal and Branched Alcohols in Benzene and n-Hexane. In: J. Chem. Thermodyn. 1, 1969, pp. 273-291, doi: 10.1016 / 0021-9614 (69) 90047-0 .
- ^ A b c V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford, 1985, p. 300.
- ↑ a b K. B. Wiberg, S. Hao: Enthalpies of hydration of alkenes. 4. Formation of acyclic tert-alcohols. In: J. Org. Chem. 56, 1991, pp. 5108-5110, doi: 10.1021 / jo00017a022 .
- ↑ GS Parks, KK Kelley, HM Huffman: Thermal data on organic compounds. V. A revision of the entropies and free energies of nineteen organic compounds. In: J. Am. Chem. Soc. 51, 1929, pp. 1969-1973, doi: 10.1021 / ja01382a003 .
- ^ HA Skinner, A. Snelson: The heats of combustion of the four isomeric butyl alcohols. In: Trans. Faraday Soc. 56, 1960, pp. 1776-1783, doi: 10.1039 / TF9605601776 .
- ↑ a b M. Caceres-Alonso, M. Costas, L. Andreoli-Ball, D. Patterson: Steric effects on the self-association of branched and cyclic alcohols in inert solvents. Apparent heat capacities of secondary and tertiary alcohols in hydrocarbons . In: Canadian Journal of Chemistry . 66 (4), 1988, pp. 989-998, doi : 10.1139 / v88-165 .
- ^ A b Thermodynamics Research Center, Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997.
- ^ A b M. Gude, AS Teja: Vapor-Liquid Critical Properties of Elements and Compounds. 4. Aliphatic Alkanols. In: J. Chem. Eng. Data . 40, 1995, pp. 1025-1036, doi: 10.1021 / je00021a001 .
- ↑ ES Domalski, ED Hearing: Heat Capacities and Entropies of Organic Compounds in the Condensed phase. Volume III. In: J. Phys. Chem. Ref. Data . 25, 1996, pp. 1-525, doi: 10.1063 / 1.555985 .
- ↑ a b c d E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ a b c Entry on butanols. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2018.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2-methylpropan-2-ol , accessed on May 1, 2020.