Sulfonal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
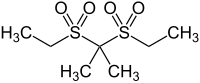
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sulfonal | |||||||||||||||
| other names |
2,2-bis (ethylsulfonyl) propane |
|||||||||||||||
| Molecular formula | C 7 H 16 O 4 S 2 | |||||||||||||||
| Brief description |
colorless, odorless and tasteless papers |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 228.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
124-126 ° C |
|||||||||||||||
| boiling point |
300 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sulfonal is a chemical compound from the group of sulfones . Sulphonal was produced by Eugen Baumann in 1888 and later introduced as a sleeping pill by Alfred Kast . Sulfonal has also been used in the treatment of the mentally ill . The sulfonals were replaced by the development of barbiturates .
Extraction and presentation
Sulfonal is produced from acetone and ethanethiol in the presence of hydrochloric acid and subsequent oxidation.
Homologues
| Sulfonals | ||||
| Surname | Sulfonal | Trional | Tetronal | |
| Basic structure |
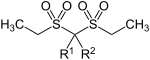
|
|||
| structure |
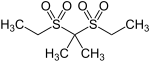
|

|

|
|
| R 1 | -CH 3 | -CH 3 | -C 2 H 5 | |
| R 2 | -CH 3 | -C 2 H 5 | -C 2 H 5 | |
| CAS number | 115-24-2 | 76-20-0 | 2217-59-6 | |
| PubChem | 8262 | 6433 | 75197 | |
| Molecular formula | C 7 H 16 O 4 S 2 | C 8 H 18 O 4 S 2 | C 9 H 20 O 4 S 2 | |
| Molar mass | 228.33 g mol −1 | 242.35 g mol −1 | 256.38 g mol −1 | |
| Melting point | 124-126 ° C | 74-76 ° C | 85 ° C | |
Individual evidence
- ↑ a b c d e f Entry on sulfonal. In: Römpp Online . Georg Thieme Verlag, accessed on March 3, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ "Sulfonal-Bayer": the new sleeping pill from Professors Baumann and Kast; the members and participants of the 61st Assembly of German Naturalists and Physicians in Cologne a. rh. presented by the paint factories, formerly Friedrich Bayer and Co.
- ↑ Hans Bangen: History of the drug therapy of schizophrenia , Berlin 1992, page 23, ISBN 3-927408-82-4 .
- ^ Otto Lueger: Sulfonal at Zeno.org .