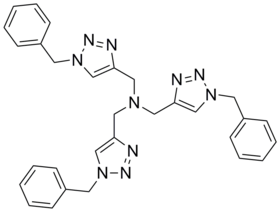
TBTA
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | TBTA | |||||||||||||||
| other names |
Tris [(1-benzyl-1 H -1,2,3-triazol-4-yl) methyl] amine |
|||||||||||||||
| Molecular formula | C 30 H 30 N 10 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 530.63 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
132-143 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
TBTA (systematic name: Tris [(1-benzyl-1 H -1,2,3-triazol-4-yl) methyl] amine) is a chemical compound from the group of triazoles (more precisely, the polytriazole amines ).
Extraction and presentation
TBTA can be obtained by reacting benzyl azide and tripropargylamine in a copper (II) acetate / sodium ascorbate solution.
use
TBTA is used in the copper- catalyzed alkyne-azide cycloaddition (CuAAC), in which TBTA prevents the disproportionation as well as the oxidation of Cu (I).
Web links
- SigmaAldrich: Click Chemistry Reagents - TBTA
- Clickchemicals: Use of Copper Ligands
Individual evidence
- ↑ Data sheet Tris ((1-benzyl-1H-1,2,3-triazol-4-yl) methyl) amine, 97% from Sigma-Aldrich , accessed on September 22, 2011 ( PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Jason E. Hein, Larissa B. Krasnova, Masayuki Iwasaki, Valery V. Fokin: Cu-Catalyzed Azide-Alkyne Cycloaddition: Preparation of Tris ((1-Benzyl-1H-1,2,3-Triazolyl) Methyl) Amine In : Organic Syntheses . 88, 2011, pp. 238-246, doi : 10.15227 / orgsyn.088.0238 ( PDF ).
- ↑ Timothy R. Chan, Robert Hilgraf, K. Barry Sharpless , Valery V. Fokin, Organic Letters, 6 (17), pp. 2853-2855, 2004 , doi : 10.1021 / ol0493094 .