Allotropic forms of oxygen
Several allotropic forms of oxygen exist .
Oxygen is a typical non-metal . In the gaseous state, it consists of individual molecules . The element forms several allotropes , which are differentiated according to the number of oxygen atoms . The most common form of oxygen is the dioxygen O 2 . More allotropes are ozone O 3 as well as the rare allotropes Tetra oxygen O 4 and Oktasauerstoff O 8 .
High pressure phases of oxygen
At room temperature and pressures greater than 10 GPa , oxygen, consisting of O 2 molecules, changes into a red solid. This phase , which apparently represents a further allotrope in addition to dioxygen (O 2 ) and ozone (O 3 ), is also called ε-oxygen or red oxygen . Based on infrared spectroscopic investigations in 1999, it was originally assumed that this was tetrasoxygen O 4 . Crystallographic analyzes of red oxygen from 2006, however, indicate new types of O 8 aggregates, which differ from the S 8 rings in sulfur . At 4800 ° C and 96 GPa there is a phase transition to ζ-oxygen , which is electrically conductive and is probably present in this metallic form inside the gas planets Jupiter and Saturn .
Tetra oxygen
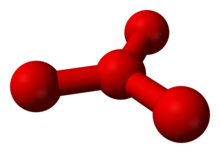
As early as 1911 there were first indications of the existence of O 4 , which was also known as oxozone and possibly originated during the ozone production according to Carl Dietrich Harries . The additions of four oxygen atoms to individual double bonds in organic compounds observed at the time suggested the temporary presence of O 4 . Tetra oxygen was also predicted by Gilbert Newton Lewis in 1924 to explain the failure of Curie's law for liquid oxygen. In fact, O 4 aggregates have already been detected in liquid oxygen. However, these are to be understood as (O 2 ) 2 , which consist of two O 2 molecules and are very unstable with a dissociation energy of 0.54 kJ / mol.
In 2001, tetrasoxygen could be indirectly detected using mass spectrometry . First, O 2 molecules and positively charged O 2 ions were combined to form O 4 ions, the existence of which could be shown by mass spectrometry. The ions were then converted into neutral O 4 molecules by absorbing electrons , which cannot be detected directly in the mass spectrometer. But since the O 4 ions reappeared after reionization , stable, neutral O 4 molecules must have existed in the meantime . Theoretical calculations so far spoke either for a triangle of oxygen atoms with a fourth atom in the center, or for a diamond-shaped molecule. The results presented also suggest that the four oxygen atoms could form two dumbbell-shaped O 2 molecules loosely bonded to one another. The assembly of the two O 2 molecules is based on the occupation of two bonding molecular orbitals (MO), which, in addition to two antibonding MOs, have arisen from four degenerate π * orbitals and are occupied by two electron pairs with antiparallel spin ( HOMO and HOMO-1) .
A possible technical application due to the high energy density of O 4 could be its use as a component in rocket fuels . It is expected that the combustion with hydrogen or hydrocarbons is even more effective than with liquid O 2 .
The differential optical absorption spectroscopy remote sensing method makes use of the fact that the collision complex O 4 occurs proportionally to the square of the known oxygen concentration. It is then possible to draw conclusions about atmospheric properties via the absorption structures of O 4 .
Octa oxygen
Based on infrared spectroscopic measurements, it has been assumed since 1999 that ε-oxygen is tetrasoxygen molecules, i.e. O 4 . However, recent crystallographic studies have shown the existence of O 8 aggregates in ε-oxygen . The O 8 structure differs from the crown-shaped S 8 rings of sulfur and resembles a somewhat compressed cube with two oxygen atoms on each of the four shortened edges. The bonds in the O 2 units are 120 picometers (pm) and thus correspond to the bond length of molecular oxygen under normal conditions (121 pm), while the distances in the O 4 rings at 219 pm are significantly smaller than that of Van der Waals -Distance of 304 pm for oxygen. The short distances of approx. 260 pm between the O 8 cubes also indicate binding interactions.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 504.
- F. Cacace, G. De Petris, A. Troiani: Experimental Detection of Tetraoxygen. In: Angewandte Chemie (International ed. In English). Volume 40, Number 21, November 2001, pp. 4062-4065, ISSN 1521-3773 . PMID 12404493 . doi : 10.1002 / 1521-3773 (20011105) 40:21 <4062 :: AID-ANIE4062> 3.0.CO; 2-X .
Web links
- Representation of the oxozone 1911 (en)
- Infrared spectrum of red oxygen (s)
- Liquid oxygen becomes a metal at high pressure. On: Wissenschaft.de of April 9, 2001
- Crystallographic analysis of the ε-oxygen (s)
- Experimental detection of octase oxygen (s)
- O 8 cluster in the ε phase of solid oxygen
Individual evidence
- ↑ Ralf Steudel , Ming Wah Wong (2007): Dark red O 8 molecules in solid oxygen: rhombus-shaped clusters instead of S 8 -like rings . Angewandte Chemie, 119 (11), 1798-1801.
- ↑ Nature News: New form of oxygen found . doi : 10.1038 / news011122-3