Thorium (III) iodide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
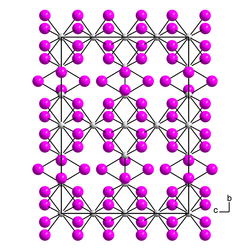
|
|||||||
| __ Th 3+ __ I - | |||||||
| Crystal system | |||||||
| Space group |
Cccm (No. 66) |
||||||
| Lattice parameters |
a = 873.5 pm |
||||||
| General | |||||||
| Surname | Thorium (III) iodide | ||||||
| other names |
Thorium triiodide |
||||||
| Ratio formula | ThI 3 | ||||||
| Brief description |
black solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 612.75 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| solubility |
reacts with water |
||||||
| Hazard and safety information | |||||||
 Radioactive |
|||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Thorium (III) iodide is a chemical compound of thorium from the group of iodides .
presentation
Thorium (III) iodide can be obtained by reacting thorium (IV) iodide with thorium at 450–550 ° C.
With a reaction time of two to three days, needle-shaped α-thorium (III) iodide is formed, while with a very long reaction time the β-form develops as compact crystals with a greenish to brass-colored sheen.
Direct representation from the elements is also possible.
properties
Thorium (III) iodide is a black, violet-tinged, mostly poorly crystallized mass. Crystals that have formed show strong dichroism from violet to olive green under the microscope and are birefringent. Above 550 ° C thorium (III) iodide breaks down into thorium (IV) iodide and thorium (II) iodide . β-Thorium (III) iodide has an orthorhombic crystal structure with the space group Cccm (space group no. 66) . The α-form has a pseudo-orthorhombic crystal structure.
Individual evidence
- ↑ HP Beck, C Strobel: ThI 3 , a Janus among the compounds with metal-metal interactions . In: Angewandte Chemie , 1982 , 94 , pp. 558-559 doi: 10.1002 / anie.19820940731 .
- ↑ a b c d e Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 1142.
- ^ RA Mackay, W. Henderson: Introduction to Modern Inorganic Chemistry, 6th edition . CRC Press, 2002, pp. 263 ( limited preview in Google Book search).
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ a b Lester R. Morss, Norman M. Edelstein, J. Fuger (Ed.): The Chemistry of the Actinide and Transactinide Elements (Set Vol.1-6 ...) . Springer, 2010, ISBN 978-94-007-0211-0 , pp. 78.84 ( limited preview in Google Book Search).
- ↑ Isabel Santos, A. Pires De Matos, Alfred G. Maddock: Compounds of thorium and uranium in low (<IV) oxidation states . In: AG Sykes (Ed.): Advances in Inorganic Chemistry . tape 34 , 1989, pp. 65–144, here p. 84 ( limited preview in Google book search).

