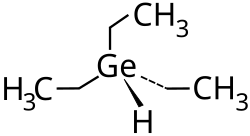
Triethyl germanium hydride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triethyl germanium hydride | |||||||||||||||
| other names |
Triethylgerman |
|||||||||||||||
| Molecular formula | (C 2 H 5 ) 3 GeH | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 160.83 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.994 g cm −3 (25 ° C) |
|||||||||||||||
| boiling point |
121-122 ° C (at 20 mmHg) |
|||||||||||||||
| Refractive index |
1.433 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Triethylgermanium hydride is a chemical compound from the group of organic germanium compounds .
Extraction and presentation
Triethyl germanium hydride can be obtained by reacting triethyl germanium chloride with lithium aluminum hydride in diethyl ether or dibutyl ether .
The reduction with lithium hydride in di- iso -pentylether is also possible, but gives a lower yield.
properties
Triethylgermanium hydride is a colorless liquid with a flash point of 8 ° C.
use
Triethylgermanium hydride is used as a catalyst for polymerizations of α- olefins . It can also be used as a cocatalyst for some reactions.
Individual evidence
- ↑ a b c d e f g h data sheet Triethylgermanium hydride, 98% from Sigma-Aldrich , accessed on October 19, 2015 ( PDF ).
- ↑ a b c Richard W. Weiss: Compounds of Germanium, Tin, and Lead, including Biological Activity and Commercial Application Covering the Literature from 1937 to 1964 . Springer Science & Business Media, 2013, ISBN 978-3-642-51889-8 , pp. 53–54 ( limited preview in Google Book search).
- ↑ John E. Drake, Christa Siebert, Bernd Wöbke: Ge Organogermanium Compounds Part 4: Compounds with Germanium-Hydrogen Bonds . Springer Science & Business Media, 2013, ISBN 978-3-662-06324-8 , pp. 14 ( limited preview in Google Book search).


![{\ displaystyle \ mathrm {4 \ ClGe (CH_ {2} CH_ {3}) _ {3} + \ LiAlH_ {4} {\ xrightarrow [{}] {Et_ {2} O}} \ 4 \ HGe (CH_ {2} CH_ {3}) _ {3} \ + \ LiCl \ + \ AlCl_ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/dc4dadaa42972ae0fc86c84821603784ae16b690)
![{\ displaystyle \ mathrm {ClGe (CH_ {2} CH_ {3}) _ {3} + \ LiH {\ xrightarrow [{}] {}} \ HGe (CH_ {2} CH_ {3}) _ {3} \ + \ LiCl \}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/306455b444c59114b7183176d6f7e4a98669e7eb)