Trioctyl phosphine oxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
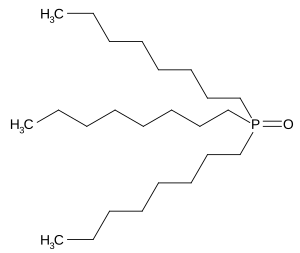
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trioctyl phosphine oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 24 H 51 OP | |||||||||||||||
| Brief description |
white, crystalline powder with a sweet smell |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 386.65 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.88 g cm −3 (15 ° C) |
|||||||||||||||
| Melting point |
48-53 ° C |
|||||||||||||||
| boiling point |
212 ° C (39.99 Pa ) |
|||||||||||||||
| solubility |
very bad in water (<1 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data |
2830 mg kg −1 ( LD 50 , rat , transdermal ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trioctylphosphine oxide , also known as trioctylphosphine oxide, is abbreviated as TOPO and is used as a high-boiling organic solvent , e.g. B. in the synthesis of cadmium selenide - semiconductor - nanocrystals .
Trioctylphosphine oxide can be made from phosphorus trichloride or from monophosphine .
See also
- Trioctylphosphine (C 8 H 17 ) 3 P

