Uranium (III) hydride
| Crystal structure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
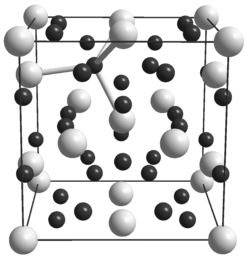
|
|||||||||
|
__ U 3+ __ D - crystal structure of β-UD 3 |
|||||||||
| General | |||||||||
| Surname | Uranium (III) hydride | ||||||||
| other names |
|
||||||||
| Ratio formula | UH 3 | ||||||||
| Brief description |
gray-brown to black solid |
||||||||
| External identifiers / databases | |||||||||
|
|||||||||
| properties | |||||||||
| Molar mass | 241.05 g mol −1 | ||||||||
| Physical state |
firmly |
||||||||
| density |
11.4 g cm −3 |
||||||||
| Melting point |
300 ° C (decomposition) |
||||||||
| solubility |
almost insoluble in water and acetone |
||||||||
| Hazard and safety information | |||||||||
 Radioactive |
|||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Uranium (III) hydride is an inorganic chemical compound of uranium from the group of hydrides .
presentation
Uranium (III) hydride can be obtained by reacting uranium with hydrogen at 150–200 ° C.
It is also formed during the oxidation of uranium in moist air at low temperatures.
The α-form can be obtained by slow reaction below -80 ° C.
The β-form is formed in a rapid reaction of finely divided uranium powder with hydrogen at 250 to 350 ° C as a fine black or dark gray powder. If massive uranium is heated in hydrogen at temperatures above 150 ° C, it forms in a strongly exothermic reaction as a gray to black, very finely divided and extremely pyrophoric powder with a maximum particle size of 4 to 5 µm. In addition to uranium dioxide, it is formed when water vapor reacts with uranium at 250 ° C.
Synthesis by reaction of uranium dioxide with calcium hydride is also possible .
properties
Uranium (III) hydride is a gray-brown to black solid that is insoluble in water. It reacts with oxygen at room temperature and with nitrogen at 250 ° C. It has a cubic crystal structure with the space group Pm 3 m (space group no. 221) . It occurs in two different modifications, whereby the α-form is irreversibly converted into the β-form at 200 ° C. Powdered uranium (III) hydride already reacts with nitrogen at 200 ° C.
use
Uranium (III) hydride is used to produce pure uranium powder through decomposition.
The use of uranium (III) hydride and uranium zirconium hydride as nuclear fuel was tested. The hydrogen contained in the material acts as a moderator on the neutrons; it slows them down, increasing the likelihood that they will split other atoms of the fuel. The thermal decomposition of the compound is said to make the reactors safer than normal reactors. However, uranium hydrides are usually undesirable by-products in nuclear fuel and nuclear waste. Tests of uranium hydride in a moderated nuclear weapon carried out in 1953 as part of Operation Upshot-Knothole proved to be a failure.
Individual evidence
- ↑ The deuterated compound was used to make it easier to determine the positions of the hydrogen atoms
- ↑ a b c d Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . Taylor & Francis US, 2011, ISBN 1-4398-1462-7 , pp. 446 f . ( limited preview in Google Book search).
- ↑ a b c d Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 784 f . ( limited preview in Google Book search).
- ↑ Entry on uranium compounds in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry uranium compounds with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling.
- ↑ a b c d Rudolf Keim: Connections with noble gases and hydrogen as well as uranium-oxygen system . Springer-Verlag, 2013, ISBN 978-3-662-10761-4 , pp. 18th ff . ( limited preview in Google Book search).
- ↑ a b Lester R. Morss, Norman M. Edelstein, J. Fuger: The Chemistry of the Actinide and Transactinide Elements (Set Vol.1-6) . Springer, 2010, ISBN 94-007-0211-6 , pp. 328–334 ( limited preview in Google Book search).
- ↑ Comprehensive Nuclear Materials: Online version . Newnes, 2011, ISBN 0-08-056033-4 ( limited preview in Google Book Search).
- ↑ D. Olander, Ehud Greenspan, Hans D. Garkisch, Bojan Petrovic: Uranium-zirconium hydride fuel properties. In: Nuclear Engineering and Design. 239, 2009, p. 1406, doi : 10.1016 / j.nucengdes.2009.04.001 .
- ^ Poston, David I .: Control of a Uranium-Hydride Reactor with Deuterium-Hydrogen Exchange . 2013, doi : 10.2172 / 1113781 .
- ↑ Nuclear Engineering International: High hopes for hydride - Nuclear Engineering International , accessed on September 9, 2017
- ↑ Self-regulating nuclear power module. In: google.com. Retrieved September 9, 2017 .
- ↑ CA Stitt, NJ Harker, KR Hallam, C. Paraskevoulakos, A. Banos, S. Rennie, J. Jowsey, TB Scott, Paul Jaak Janssen: An Investigation on the Persistence of Uranium Hydride during Storage of Simulant Nuclear Waste Packages. In: PLOS ONE. 10, 2015, p. E0132284, doi : 10.1371 / journal.pone.0132284 .
- ^ Lillian Hoddeson, Paul W. Henriksen, Roger A. Meade, Catherine L. Westfall: Critical Assembly A Technical History of Los Alamos During the Oppenheimer Years, 1943-1945 . Cambridge University Press, 2004, ISBN 978-0-521-54117-6 , pp. 181 ( limited preview in Google Book search).




