Vanoxerine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
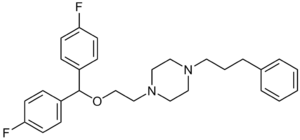
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Vanoxerine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 28 H 32 F 2 N 2 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class |
Dopamine reuptake inhibitors |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 450.56 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Vanoxerine , also known as GBR-12909 known is a piperazine - derivative , which in humans as a selective dopamine reuptake inhibitor acts (SDRI). It was developed as a substitute for the treatment of cocaine addicts in order to alleviate the psychological withdrawal symptoms . GBR-12909 binds the dopamine re -uptake transporter like cocaine , but 100 times stronger without having a notable stimulating effect, since in contrast to cocaine it prevents the simultaneous release of dopamine. The half-life of vanoxerine is also much longer than that of cocaine.
Vanoxerin has been in the second phase since 2006; that is, experiments are made on humans.
There was also a capric acid - ester of a hydroxy-Vanoxerins developed (DBL-583) serving as custodian syringe can be given and about acting for a month.
Individual evidence
- ↑ Data sheet GBR 12909 dihydrochloride from Sigma-Aldrich , accessed on December 14, 2012 ( PDF ).
- ↑ Differential Reinforcing Effects of Cocaine and GBR-12909: Biochemical Evidence for Divergent Neuroadaptive Changes in the Mesolimbic Dopaminergic System The Journal of Neuroscience, December 1, 1996, 16 (23): 7416-7427 (PDF; 884 kB).