Vilazodon
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
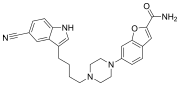
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Vilazodon | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 26 H 27 N 5 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| properties | |||||||||||||
| Molar mass | 441.52 g mol −1 (free base) | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Vilazodone is the international non-proprietary name for an antidepressant effective drug . The drug was on 21 January 2011 by the US Food and Drug Administration (FDA) as a drug under the brand name Viibryd approved . The substance requires a prescription; the medicinal product used is vilazodon hydrochloride .
Mode of action
Vilazodon works in two ways. It is primarily a selective serotonin reuptake inhibitor and it also acts as a partial agonist selectively on the 5-HT 1A receptor . The affinity for the other 5-HT receptors, on the other hand, is very low. This working principle is unique to date. It combines the mechanisms of action of the hitherto conventional first-line treatment ( first-line therapy ) with the second-line treatment ( second-line therapy ).
Side effects
Vilazodon is generally well tolerated. Major side effects include diarrhea , nausea, and headache . In contrast to other antidepressants, vilazodon does not appear to have any influence on the patient's body weight or sexual functions (see also: Serotonin reuptake inhibitors # Adverse effects ).
Development history
Vilazodon was originally developed by Merck KGaA and patented in 1993. The market entry was planned for 2005. The initial maximum dose was 20 mg. In February 2001, Merck licensed GlaxoSmithKline . Both companies wanted to develop Vilazodon together and market it after approval. The substance went through 15 phase I and 5 phase II studies, in which no reliable evidence of significant efficacy could be provided. In September 2004, Merck then granted exclusive worldwide rights to the US biotechnology company Genaissance Pharmaceuticals . In June 2005, Genaissance Pharmaceuticals was acquired by Clinical Data , Inc. From February 2006 to May 2007 Genaissance Pharmaceuticals conducted a phase III study with vilazodon to determine the efficacy and possible genetic markers.
The company Trovis Pharmaceuticals, LLC (formerly PGxHealth, LLC ), a 100% owned subsidiary of Clinical Data, led from March 2008 to March 2009, a randomized placebo-controlled double- blind study (Phase III) by using Vilazodone and a dose of 40 mg of active ingredient. An open phase III study ran from December 2007 to May 2009. These studies provided evidence of effectiveness, which led to the approval of vilazodone by the FDA.
In April 2011, Clinical Data was acquired by Forest Laboratories .
Pharmacological properties
Vilazodone is orally administered and has a plasma half-life of 20-24 hours. The bioavailability is around 72%.
further reading
- MP Cruz: Vilazodone HCl (Viibryd): A Serotonin Partial Agonist and Reuptake Inhibitor For the Treatment of Major Depressive Disorder. In: P & T. Volume 37, Number 1, January 2012, pp. 28-31, PMID 22346333 , PMC 3278186 (free full text).
- A. Khan, AJ Cutler, DK Kajdasz, S. Gallipoli, M. Athanasiou, DS Robinson, H. Whalen, CR Reed: A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. In: The Journal of clinical psychiatry Volume 72, Number 4, April 2011, pp. 441-447, doi : 10.4088 / JCP.10m06596 . PMID 21527122 .
- LA Dawson, JM Watson: Vilazodone: a 5-HT1A receptor agonist / serotonin transporter inhibitor for the treatment of affective disorders. In: CNS Neuroscience & Therapeutics Volume 15, Number 2, 2009, pp 107-117, PMID 19499624 . (Review).
- R. Adamec, GD Bartoszyk, P. Burton: Effects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. In: European Journal of Pharmacology Volume 504, Number 1-2, November 2004, pp. 65-77, doi : 10.1016 / j.ejphar.2004.09.009 . PMID 15507223 .
- T. Heinrich, H. Böttcher, R. Gericke, GD Bartoszyk, S. Anzali, CA Seyfried, HE Greiner, C. Van Amsterdam: Synthesis and structure-activity relationship in a class of indolebutylpiperazines as dual 5-HT (1A) receptor agonists and serotonin reuptake inhibitors. In: Journal of Medicinal Chemistry Volume 47, Number 19, September 2004, pp. 4684-4692, doi : 10.1021 / jm040793q . PMID 15341484 .
- D. Treit, A. Degroot, S. Kashluba, GD Bartoszyk: Systemic EMD 68843 injections reduce anxiety in the shock-probe, but not the plus-maze test. In: European journal of pharmacology Volume 414, Number 2-3, March 2001, pp. 245-248, PMID 11239925 .
- H. Murck, RM Frieboes, IA Antonijevic, A. Steiger: Distinct temporal pattern of the effects of the combined serotonin-reuptake inhibitor and 5-HT1A agonist EMD 68843 on the sleep EEG in healthy men. In: Psychopharmacology Volume 155, Number 2, May 2001, pp. 187-192, PMID 11401008 .
- EA Rabiner, RN Gunn, MR Wilkins, PA Sargent, E. Mocaer, E. Sedman, PJ Cowen, PM Grasby: Drug action at the 5-HT (1A) receptor in vivo: autoreceptor and postsynaptic receptor occupancy examined with PET and [ carbonyl- (11) C] WAY-100635. In: Nuclear medicine and biology Volume 27, Number 5, July 2000, pp. 509-513, PMID 10962259 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ fda.gov: FDA Approved Drug Products.Retrieved May 20, 2011.
- ↑ K. Traynor: Vilazodone approved for major depression. In: American Journal of Health-System Pharmacy Volume 68, Number 5, March 2011, p. 366, doi : 10.2146 / news110009 . PMID 21330672 .
- ↑ fda.gov: Application Number: 022567 - Orig1s000 Proprietary Name Review (s). dated November 15, 2010.
- ^ GD Bartoszyk, R. Hegenbart, H. Ziegler: EMD 68843, a serotonin reuptake inhibitor with selective presynaptic 5-HT1A receptor agonistic properties. In: European journal of pharmacology Volume 322, Number 2-3, March 1997, pp. 147-153, PMID 9098681 .
- ↑ a b M. E. Page, JF Cryan, A. Sullivan, A. Dalvi, B. Saucy, DR Manning, I. Lucki: Behavioral and neurochemical effects of 5- (4- [4- (5-cyano-3-indolyl) -butyl) -butyl] -1-piperazinyl) -benzofuran-2-carboxamide (EMD 68843): a combined selective inhibitor of serotonin reuptake and 5-hydroxytryptamine (1A) receptor partial agonist. In: Journal of pharmacology and experimental therapeutics Volume 302, number 3, September 2002, pp. 1220-1227, doi : 10.1124 / jpet.102.034280 . PMID 12183683 . free full text
- ↑ a b R. H. Howland: Vilazodone: Another novel atypical antidepressant drug. In: Journal of psychosocial nursing and mental health services Volume 49, Number 3, March 2011, pp. 19-22, doi : 10.3928 / 02793695-20110203-98 . PMID 21323263 .
- ↑ Edgar Filing: CLINICAL DATA INC - Form 10-K. From June 19, 2007 p. 30.
- ↑ reuters.com: FDA approves Clinical Data Inc's antidepressant. January 21, 2011.
- ↑ H. Bottcher, C. Seyfried et al: US Patent 5,532,241
- ↑ Focus on value and time to market: Clinical development pipeline set to fuel sustainable growth. Press release from Merck KGaA on October 26, 2000.
- ↑ Center for Drug Evaluation and Research: APPLICATION NUMBER: 022567Orig1s000. (PDF; 2.1 MB) p. 1. Retrieved on May 21, 2011.
- ↑ GSK fills product gap by licensing Merck KGaA antidepressant. dated February 16, 2001.
- ↑ chemie.de: GlaxoSmithKline and Merck KGaA sign a worldwide license agreement for a novel antidepressant. 16 February 2001.
- ↑ Edgar Filing: CLINICAL DATA INC - Form 10-K. From June 19, 2007 p. 31.
- ↑ Edgar Filing: CLINICAL DATA INC - Form 10-K. From June 19, 2007 p. 65.
- ↑ Merck KgaA licenses out Vilazodone to Genaissance Pharmaceuticals. In: Handelsblatt of September 23, 2004.
- ↑ Clinical Data to Aquire Genaissance Pharmaceuticals. ( Memento from December 22, 2014 in the Internet Archive ) (PDF; 54 kB) Press release from June 21, 2005.
- ↑ clinicaltrials.gov: Effectiveness Study of Vilazodone to Treat Depression and to Discover Genetic Markers Associated With Response. Retrieved May 20, 2011.
- ^ United States Securities and Exchange Commission: Form 8-K Current Report; CLINICAL DATA, INC. January 21, 2011.
- ↑ clinicaltrials.gov: Randomized, Double-Blind, Placebo Controlled Study of Vilazodone's Efficacy, Safety, and Biomarkers of Response in Major Depressive Disorder (MDD). Retrieved May 20, 2011.
- ↑ clinicaltrials.gov: A One Year Open Label Study Assessing the Safety and Tolerability of Vilazodone in Patients With Major Depressive Disorder (MDD). Retrieved May 20, 2011.
- ↑ Forest Laboratories wants to take over Clinical Data. ( Memento from March 16, 2011 in the Internet Archive ) In: Ärzte Zeitung from March 9, 2011.
- ↑ finanznachrichten.de: Forest Laboratories Completes Acquisition of Clinical Data, Inc .. of April 14, 2011.
- ↑ Center for Drug Evaluation and Research: APPLICATION NUMBER: 022567Orig1s000. (PDF; 2.1 MB) Retrieved May 21, 2011.