Zeta potential
The zeta potential (also known as the potential) is the electrical potential (also referred to as Coulomb potential) at the shear layer of a moving particle in a suspension or emulsion.
The electric potential describes the ability of a (from a charge induced) field , apply force to other loads; the difference in electrical potential at two locations is the electrical voltage .
root cause
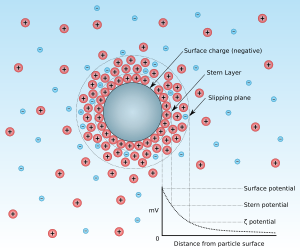
If charged particles are in suspension, ions of the suspension medium are deposited on the particle surface in a firmly bound Helmholtz double layer . Further ions are rather loosely bound in a diffuse, i.e. H. disorderly, layer at. The particle appears electrically neutral from a great distance, because all charges or potentials of the particle are compensated by ions of the suspension medium.
If a particle moves, part of the loosely bound diffuse layer is sheared off by friction and the particle no longer appears electrically neutral, but has a potential again. This potential at the shear limit is called the zeta potential. With the same medium, it is therefore a relative measured variable for the surface potential and thus for the charge of the particle. The zeta potential can be measured by moving the charged particle through an applied electric field. The resulting speed is then a measure of the zeta potential.
The electrophoretic mobility can be calculated with the help of the zeta potential :
With
- the dielectric constant of the sample
- the Henry function (Hückel and Smoluchowski approximation); it takes values between 1 (Hückel approximation, non-polar solvent) and 1.5 (Smoluchowski, polar solvent)
- the dynamic viscosity of the liquid.
Connections
- A forming electrical charges at interfaces can also by friction or thermal movement effected
- Positively charged ions (counter ions) are attracted (outer Helmholtz layer) and electrical double layers are created
- Further ions have a loose arrangement that extends into the liquid phase (diffuse layer). Molecular or thermal movement results in a diffuse distribution of positive and negative ions, with ions with the same charge repelling one another. The total charge is balanced here.
- The shear forces that occur when the particles move in the liquid do not affect the ion layer that is firmly bound to the surface ( shear plane). Since the electrostatic attraction between the charged surface and the diffuse layer decreases with increasing distance, the concentration of the initially still predominant counterions decreases with distance, and finally negative and positive ions are evenly present in the neutral zone.
- The true charge of the particles is characterized by the difference between the potentials on the particle surface ( Nernst potential ) and in the neutral zone and therefore cannot be determined experimentally.
- The zeta potential is also called electrokinetic potential : potential difference of the diffuse layer; characterizes the repulsion energy between the particles (depending on the product of distance and the reciprocal of the effective radius of the electrical double layer)
- An increase in the zeta potential increases the interparticle repulsion forces, so that aggregations are reduced or even prevented.
measuring technology
Electroacoustics
An alternating electric field is applied to the dispersion between two electrodes. It generates a sound wave of the same frequency, similar to the piezoelectric effect of crystals. Is measured electrokinetic sound amplitude ( electrokinetic sound amplitude , ESA).
The ESA method for characterizing the charge stability of particles in a dispersion is an electroacoustic measurement technique. An oscillating voltage is applied to a suspension, dispersion or emulsion which is generated by an alternating current source. Charged particles in the dispersion vibrate at the frequency of the externally applied electric field. One or more frequencies can be applied. The oscillation of the particles at these frequencies creates sound waves. The amplitudes of these sound waves are measured as electrokinetic sound amplitude (ESA). This ESA signal is proportional to the dynamic mobility of the particles and this in turn is proportional to the zeta potential of the particles present in the dispersion. A prerequisite for using this effect is a certain density difference between the dispersion medium and the particles. In order to generate evaluable signals, this density difference must be at least 0.2 g / cm³.
Streaming potential
In principle, streaming potential measurements take place in such a way that the solid particles are compressed to form a diaphragm, in which a liquid flowing at a constant speed then experiences a pressure drop. The movement of the liquid causes part of the electrochemical double layer to be sheared off, creating a potential difference that is picked up by measuring electrodes. This voltage, which is tapped at both electrodes, is proportional to the zeta potential of the particles. After calibration with standard materials, the measurement signal is output as a streaming potential or zeta potential .
Electrophoresis
The electrophoretic mobility of the particles in a dispersion allows conclusions to be drawn about the zeta potential of the particles in a dispersion. A suitable method to measure this is that of the laser Doppler effect. Laser Doppler anemometry is used to measure the zeta potential of particles. There are measuring devices that use the photocorrelation spectroscopy PCS as well as the laser Doppler anemometry (LDA). This allows the particle sizes and the zeta potential in a dispersion to be determined.
Web links
literature
- Robert J. Hunter: Zeta potential in colloid science: principles and applications. Academic Press, London 1981, ISBN 0-12-361960-2 .
Individual evidence
- ↑ Gerhard Lagaly, Oliver Schulz, Ralf Zimehl: Dispersions and emulsions: An introduction to the colloidics of finely divided substances including clay minerals . Ed .: Klaus Beneke. Steinkopff, Heidelberg 1997, ISBN 3-642-59248-1 , urn : nbn: de: 1111-201109214107 .
- ↑ Volker Ender: Practical course in physical chemistry. Springer Verlag, ISBN 978-3-662-45470-1 (eBook).