Zinc dithionite
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
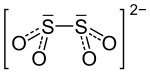
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Zinc dithionite | |||||||||||||||
| other names |
Zinc hydrosulfite |
|||||||||||||||
| Molecular formula | ZnS 2 O 4 | |||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 193.45 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Zinc dithionite (chemical formula ZnS 2 O 4 ) is produced from sulfur dioxide or hydrogen sulfites through reduction with zinc .
Extraction and presentation
It is produced by introducing sulfur dioxide into a mixture of water and zinc dust.
or
use
Zinc dithionite is used as a bleaching agent in the textile and paper industry. Contact with oxygen must be avoided, as it is oxidized by oxygen and becomes ineffective.
For ecological reasons (no pollution of the sewage with zinc salts) it is increased u. a. replaced by sodium dithionite .
Individual evidence
- ↑ data sheet .
- ↑ data sheet at NOAA (English) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Martin BB Hocking: Handbook of Chemical Technology and Pollution Control, 3rd Edition. Academic Press, 2006, ISBN 978-0-080-47827-2 , p. 485 ( limited preview in Google Book Search).
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry . 2nd ed., Vol. 1, Academic Press 1963, pp. 394-395.
- ↑ bleach (peroxygen chemicals) .


