Tin (II) selenide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
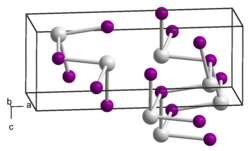
|
||||||||||||||||
| __ Sn 2+ __ Se 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Tin (II) selenide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | SnSe | |||||||||||||||
| Brief description |
steel-gray solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 197.67 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.18 g cm −3 |
|||||||||||||||
| Melting point |
861 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tin (II) selenide is an inorganic chemical compound of tin from the group of selenides .
Extraction and presentation
Tin (II) selenide can be obtained by reacting tin with selenium at 350 ° C.
However, there are also known other ways of presenting organotin compounds.
properties
Tin (II) selenide is a steel-gray solid that is insoluble in water. The compound occurs in two crystal structures , the normal temperature variant of which has an orthorhombic crystal structure (a = 11.50 Å, b = 4.15 Å and c = 4.44 Å).
use
Tin (II) selenide is a (IV-VI) semiconductor with a narrow band gap and is of great interest in areas such as low-cost photovoltaics and storage switching devices.
Individual evidence
- ^ A b Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 1995, pp. 385 ( limited preview in Google Book Search).
- ↑ a b c d data sheet Tin (II) selenide, 99.995% trace metals basis from Sigma-Aldrich , accessed on October 13, 2014 ( PDF ).
- ↑ a b c d e data sheet Tin selenide, 99.999% (metals basis) from AlfaAesar, accessed on October 13, 2014 ( PDF )(JavaScript required) .
- ↑ R. Colin, J. Drowart: Thermodynamic study of tin selenide and tin tellurides using a mass spectrometer. In: Transactions of the Faraday Society. 60, 1964, p. 673, doi : 10.1039 / TF9646000673 .
- ^ Egon Wiberg, Nils Wiberg: Inorganic Chemistry . Academic Press, 2001, pp. 906 ( limited preview in Google Book search).
- ^ Alan H. Cowley: Inorganic Syntheses . John Wiley & Sons, 2009, ISBN 0-470-13297-3 , pp. 86 ( limited preview in Google Book search).
- ↑ Y. Feutelais, M. Majid, B. Legendre, SG FRICS: Phase diagram investigation and proposition of a thermodynamic evaluation of the Tin-selenium system. In: Journal of Phase Equilibria. 17, 1996, pp. 40-49, doi : 10.1007 / BF02648368 .
- ↑ N. kumar, U. Parihar, R. Kumar, KJ Patel, CJ Panchal, N. Padha: Effect of Film Thickness on Optical Properties of Tin Selenide Thin Films Prepared by Thermal Evaporation for Photovoltaic Applications. In: American Journal of Materials Science. 2, 2012, pp. 41-45, doi : 10.5923 / j.materials.20120201.08 .



