1,2,3-triazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,2,3-triazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 3 N 3 | |||||||||||||||
| Brief description |
colorless liquid (from 25 ° C) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 69.07 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.192 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
23-25 ° C |
|||||||||||||||
| boiling point |
203 ° C (1003 hPa) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| Refractive index |
1.4854 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,2,3-Triazole is a chemical compound from the group of the triazoles and isomeric to 1,2,4-Triazole .
Extraction and presentation
1,2,3-Triazole can be obtained by Huisgen azide-alkyne 1,3-dipolar cycloaddition , copper (CuAAC) or ruthenium (RuAAC) catalyzed azide-alkyne cycloaddition .
properties
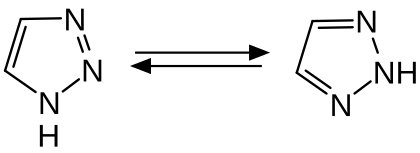
1,2,3-triazole is a colorless liquid with a pK s value of 1.17 weak base is. Two tautomers can occur with the 1 H and 2 H forms . In solution, the 2 H form dominates in most solvents .
The compound can decompose strongly exothermic. A thermoanalytical investigation showed a decomposition reaction with a very high heat of reaction of −2600 kJ / kg (−180 kJ / mol) from 200 ° C. Despite the high heat of decomposition, the connection is not explosive in terms of the Explosives Act , as no positive result was observed either in the BAM 50/60 steel pipe test or in the Koenen test with a 2 mm hole diameter. The Koenentest with 1 mm hole diameter is positive.
use
1,2,3-Triazole is used in research as a building block for more complex chemical compounds, such as B. used the active ingredient tazobactam .
Individual evidence
- ↑ a b c d e f data sheet 1,2,3-triazole from Sigma-Aldrich , accessed on March 5, 2011 ( PDF ).
- ↑ Entry on 1,2,3-1H-triazoles at ChemBlink , accessed on March 5, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-488.
- ↑ Organic Chemistry Portal: Synthesis of 1,2,3-triazoles and benzotriazoles
- ^ Theophil Eicher, Siegfried Hauptmann, Andreas Speicher: The chemistry of heterocycles: structure, reactions, syntheses, and applications , Chapter: 123-Triazole, p. 201; ISBN 978-3-5273-0720-3 ( limited preview in Google Book Search).
- ↑ a b c d M. Malow, KD Wehrstedt, S. Neuenfeld: On the explosive properties of 1H-benzotriazole and 1H-1,2,3-triazole . Tetrahedron Letters 48 (2007), 1233-1235, doi : 10.1016 / j.tetlet.2006.12.046 .