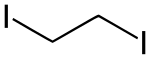
1,2-diiodoethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,2-diiodoethane | |||||||||||||||
| Molecular formula | C 2 H 4 I 2 | |||||||||||||||
| Brief description |
yellow monoclinic prisms |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 281.86 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.325 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
83 ° C |
|||||||||||||||
| boiling point |
200 ° C |
|||||||||||||||
| solubility |
soluble in ethanol , diethyl ether , acetone and chloroform |
|||||||||||||||
| Refractive index |
1,871 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
9.3 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,2-Diiodoethane is a doubly iodinated derivative of ethane and thus a halogenated hydrocarbon . It is isomeric to 1,1-diiodoethane .
properties
1,2-Diiodoethane forms yellow monoclinic prisms with a melting point of 83 ° C and a high density of 3.325 g · cm −3 . The critical point is at a temperature of 749.91 K , a pressure of 47.30 bar and a volume of 323.5 ml · mol −1 .
use
In organic synthesis it is mainly used to prepare samarium (II) iodide and ytterbium (II) iodide in THF .
Individual evidence
- ↑ a b c d e f David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 3-184.
- ↑ a b data sheet 1,2-diiodoethane from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ^ Carl L. Yaws: "Thermophysical properties of chemicals and hydrocarbons", p. 6 ( limited preview in the Google book search).
- ↑ P. Girard, Jean-Louis Namy, Henri B. Kagan : "Divalent Lanthanide Derivatives in Organic Synthesis. 1. Mild Preparation of SmI 2 and YbI 2 and Their Use as Reducing or Coupling Agents", in: J. Am. Chem. Soc. , 1980 , 102 (8) , pp. 2693-2698 ( doi : 10.1021 / ja00528a029 ).

![{\ displaystyle \ mathrm {Sm \ + \ ICH_ {2} CH_ {2} I \ {\ xrightarrow [{}] {THF}} \ SmI_ {2} \ + \ H_ {2} C {=} CH_ {2 }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b5fa13ca03a09e925e5d0cce8e670d26f6356b8a)