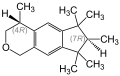1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta ( g ) -2-benzopyran
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
![Structural formula of 4,6,6,7,8,8-hexamethyl-1,3,4,6,7,8-hexahydrocyclopenta [g] isochromes](https://upload.wikimedia.org/wikipedia/commons/thumb/4/4d/Galaxolide_200.svg/214px-Galaxolide_200.svg.png)
|
||||||||||||||||
| Structural formula of the main component (without specifying the stereochemistry ) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta [ g ] -2-benzopyran | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 26 O | |||||||||||||||
| Brief description |
viscous liquid with a musky odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 258.40 g mol −1 | |||||||||||||||
| Vapor pressure |
72.7 mPa (25 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (1.75 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta [ g ] -2-benzopyran is a mixture of several chemical compounds , which is one of the polycyclic musk compounds and is used as a fragrance .
The abbreviation HHCB is used on the one hand for the substance itself or on the other hand for mixtures of substances consisting of several polycyclic musk compounds and the main component of which is 1,3,4,6,7,8- H exahydro-4,6,6,7,8 , 8 h examethyl- c yclopenta [ g ] -2- b enzopyran is.
composition
The following composition has been described for HHCB as a substance mixture:
- 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta [ g ] -2-benzopyran (74-76%)
- 1,3,4,6,7,8-hexahydro-4,6,8,8-tetramethyl-6-ethyl-cyclopenta [ g ] -2-benzopyran or
- 1,3,4,6,7,8-hexahydro-4,6,6,8-tetramethyl-8-ethyl-cyclopenta [ g ] -2-benzopyran (together 6-10%)
- 1,3,4,7,8,9-hexahydro-4,7,7,8,9,9-hexamethyl-cyclopenta [ h ] -2-benzopyran (5-8%)
- 1,2,4,7,8,9-hexahydro-1,7,7,8,9,9-hexamethyl-cyclopenta [ f ] -2-benzopyran (6-8%)
- Stereoisomers of the main component 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta [ g ] -2-benzopyran
The main component (74–76%) contains two stereocenters in the 4- and 7-positions, so there are four stereoisomers , the (4 S , 7 R ) form, the (4 R , 7 S ) form, the ( 4 R , 7 R ) form and the (4 S , 7 S ) form with the empirical formula C 18 H 26 O:
Studies have shown that the (4 S , 7 R ) shape and the (4 S , 7 S ) shape of the main component are crucial for the musk odor note, with very low odor thresholds of 1 ng / L or less. The three secondary components with 5–10% each - a total of 24–26% - each also contain stereocenters, i.e. they are complex mixtures of substances.
use
HHCB is used as a fragrance in detergents and cleaning agents as well as in cosmetic products. In addition to HHCB, AHTN (7-acetyl-1,1,3,4,4,6-hexamethyltetralin) is one of the most important representatives of polycyclic musk compounds in terms of quantity, the consumption of which in the EU and the USA is over 1000 tons annually .
Web links
- Opinion of the SCCNFP concerning Hexahydro-hexamethyl-cyclopenta (γ) -2-benzopyran (HHCB) from September 17, 2002.
Individual evidence
- ↑ aromatic galaxolide
- ↑ a b c Entry on HHCB. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ a b Entry on 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylindeno (5,6-c) pyran in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylindeno [5,6-c] pyran in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ G. Frater, U. Mueller, P. Kraft, Helv. Chim. Acta, 82 (10), 1656-1665 (1999).
- ↑ Entry on polycyclic musk compounds. In: Römpp Online . Georg Thieme Verlag, accessed on June 7, 2014.