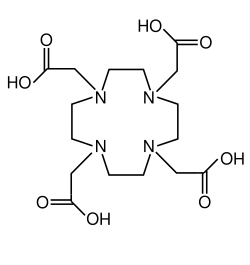
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 28 N 4 O 8 | ||||||||||||||||||
| Brief description |
light yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 404.42 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid , usually just referred to as DOTA , is a four-protonic acid and is chemically related to ethylenediaminetetraacetic acid (EDTA). DOTA is used in medicine as a chelating complexing agent.
synthesis
DOTA is most easily made by reacting Cyclen (1,4,7,10-tetraazacyclododecane) with four equivalents of bromoacetic acid. In this way, DOTA was first synthesized in 1976 by Hermann Stetter and Wolfram Frank at the Institute for Organic Chemistry at the TH Aachen .
use
The excellent complex formation properties of divalent and trivalent metal cations make DOTA ideal for use in medicine . Both in diagnostics and in therapy , DOTA or derivatives of DOTA (so-called conjugates ) are used. For example, the 1: 1 complex of Gd 3+ and DOTA is used for transport as a contrast agent in MRI examinations. The compound has the international non-proprietary name Gadoteric acid (brand names Dotarem, Multihance, Gadovist ).
In nuclear medicine , the compound DOTATOC , a DOTA-tyrosine conjugate with octreotide , is used for the diagnosis and treatment of metastatic neuroendocrine tumors . For diagnostics, for example, the DOTA is loaded with the isotope 68 gallium, for therapy with β emitters such as 90 yttrium. DOTA is the most frequently used chelator in nuclear medicine, as it forms complexes with many subgroup and transition metals of extremely high stability.
literature
- C. Wängler et al. a .: Improved syntheses and applicability of different DOTA building blocks for multiply derivatized scaffolds. In: Bioorganic & Medicinal Chemistry 16/2008, pp. 2606-16.
- B. Yooa, MD Pagel: A facile synthesis of greek small letter alpha-amino-DOTA as a versatile molecular imaging probe. In: Tetrahedron Letters 47/2006, pp. 7327-30
- LH Bryant et al. a .: Pharmacokinetics of a High-Generation Dendrimer-Gd-DOTA. In: Academic Radiology 9/2002, pp. 29-33.
- RS Ranganathan et al. a .: Polymethylated DOTA ligands. 1. Synthesis of rigidified ligands and studies on the effects of alkyl substitution on acid-base properties and conformational mobility. In: Inorg. Chem. 41/2002, pp. 6846-55. PMID 12470083
- U. Cosentino et al. a .: Conformational characterization of lanthanide (III) -DOTA complexes by ab initio investigation in vacuo and in aqueous solution. In: J. Am. Chem. Soc. 124/2002, pp. 4901-9. PMID 11971741
- MF Tweedle: Gadolinium chelates as relaxation agents in magnetic resonance imaging. In: Journal of Alloys and Compounds 180/1992, pp. 317-23.
- M. Ginja, HR Maecke: Synthesis of trifunctional somatostatin based derivatives for improved cellular and subcellular uptake. In: Tetrahedron Letters 46/2005, pp. 2821-4
- P. Winter u. a .: Improved paramagnetic chelate for molecular imaging with MRI. In: Journal of Magnetism and Magnetic Materials 293/2005, pp. 540-5.
Individual evidence
- ↑ a b c data sheet 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid from Sigma-Aldrich , accessed on October 23, 2016 ( PDF ).
- ↑ B. Wängler et al. a .: Application of tris-allyl-DOTA in the preparation of DOTA-peptide conjugates. In: Tetrahedron Letters 57/2006, pp. 5985-5988.
- ↑ S. Knör u. a .: Synthesis of novel 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) derivatives for chemoselective attachment to unprotected polyfunctionalized compounds. In: Chemistry 13/2007, pp. 6082-6090. PMID 17503419 .
- ↑ H. Stetter, W. Frank: Complex Formation with Tetraazacycloalkane-N, N ', N' ', N' '' - tetraacetic Acids as a Function of Ring Size. In: Angewandte Chemie International Edition Volume 15, Number 11, 1976, p. 686. doi : 10.1002 / anie.197606861 .
- ↑ C. Wängler: Synthesis, characterization and evaluation of antibody conjugates with dendrimer-based chelator and fluorescent dye multimers for cancer diagnosis and therapy. Dissertation, Ruprecht-Karls-Universität Heidelberg, 2007.
