Cyclen
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
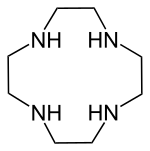
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclen | |||||||||||||||
| other names |
1,4,7,10-tetraazacyclododecane |
|||||||||||||||
| Molecular formula | C 8 H 20 N 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 172.27 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
110-113 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cyclen , or 1,4,7,10-tetraazacyclododecane , is a cyclic polyamine . Cyclen is the starting material for DOTA and DOTA derivatives , which are widely used as medicinal products .
synthesis
Cyclen can be made by combining two nucleophilic substitution reactions . The synthesis first described by Richman and Atkins in 1974 can also be used to prepare many related macrocyclic polyazaalkanes.
The starting product is diethylenetriamine (1), which is converted to (3) with 3 equivalents of tosyl chloride in pyridine . In sodium ethoxide , one proton each of the two primary amines of diethylenetriamine is split off and replaced by sodium ions (4). Diethanolamine (2) is used as the second starting compound. It is also converted to (5) with 3 equivalents of tosyl chloride. Both compounds (4) and (5) are made to react in great dilution in dimethylformamide in order to avoid polymerization into long chains. In the last step the tosyl groups are removed with sulfuric acid and the cyclen (7) is obtained. The crude product is mostly slightly yellow in color due to impurities. Cyclen can be obtained very pure as a white solid by sublimation .
use
Cyclen has excellent complexing properties. Like crown ethers, cations are selectively bound.
A large part of the cycle is processed further technically and reacted with bromoacetic acid in order to obtain DOTA or DOTA derivatives. Since the nitrogen atoms, in contrast to the oxygen atoms of the crown ethers, allow a further bond, similar to DOTA, (several) larger radicals, such as B. adenosine, coupled to obtain new properties. These very strong complexing agents are used in a variety of ways both in diagnostics and in therapy .
literature
- C. Wängler, B. Wängler, M. Eisenhut, U. Haberkorn, W. Mier: Improved syntheses and applicability of different DOTA building blocks for multiply derivatized scaffolds , in: Bioorganic & Medicinal Chemistry , 2008 , 16 (5), p. 2606-2016; PMID 18065226 .
- R. Delgado, V. Félix, LM Lima, DW Price: Metal complexes of cyclen and cyclam derivatives useful for medical applications: a discussion based on thermodynamic stability constants and structural data , in: J. Chem. Soc., Dalton Trans. , 2007 , 26 , pp. 2734-2745; PMID 17592589 .
- Julien Massue, Sally E. Plush, Célia S. Bonnet, Doireann A. Moore and Thorfinnur Gunnlaugsson: Selective mono N-alkylations of cycles in one step syntheses , in: Tetrahedron Letters , 2007 , 48 , pp. 8052-8055; doi : 10.1016 / j.tetlet.2007.09.022 .
- XY Tan, J. Zhang, Y. Huang, Y. Zhang, LH Zhou, N. Jiang, HH Lin, N. Wang, CQ Xia, XQ Yu: Synthesis and DNA-cleavage properties of metal complexes of 1,4,7 , 10-tetraazacyclododecane (cyclen) functionalized with a pendant benzocrown ether , in: Chem. Biodivers. , 2007 4 (9), pp. 2190-2197; PMID 17886837 .
- E. Delgado-Pinar, JC Frías, LJ Jiménez-Borreguero, MT Albelda, J. Alarcón, E. García-España: One-pot preparation of surface modified boehmite nanoparticles with rare-earth cycle complexes , in: Chem. Commun. (Camb.) , 2007 , 32 , pp. 3392-3394; PMID 18019508 .
- KE Borbas, JI Bruce: Synthesis of asymmetrically substituted cyclen-based ligands for the controlled sensitization of lanthanides , in: Org. Biomol. Chem. , 2007 , 5 , pp. 2274-2282; PMID 17609759 .
- Katell Sénéchal-David, Simon JA Pope, Susan Quinn, Stephen Faulkner, Thorfinnur Gunnlaugsson: Sensitized Near-Infrared Lanthanide Luminescence from Nd (III) - and Yb (III) -Based Cyclen − Ruthenium Coordination Conjugates , in: Inorg. Chem. , 2006 , 45 (25), pp. 10040-10042; doi : 10.1021 / ic061706i .
Individual evidence
- ↑ Data sheet 1,4,7,10-Tetraazacyclododecane (PDF) from Strem, accessed on December 25, 2012.
- ↑ Data cycles at Sigma-Aldrich , accessed on 23 March 2011 ( PDF ).
- ↑ a b Entry on 1,4,7,10-tetraazacyclododecane in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on 1,4,7,10-tetraazacyclododecane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 15, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ JE Richman, TJ Atkins: Nitrogen analogs of crown ethers , in: J. Am. Chem. Soc. , 1974 , 96 , pp. 2268-2270; doi : 10.1021 / ja00814a056 .



