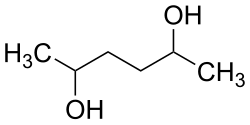
2,5 hexanediol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| Simplified structural formula without stereochemistry | ||||||||||
| General | ||||||||||
| Surname | 2,5 hexanediol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 6 H 14 O 2 | |||||||||
| Brief description |
colorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 118.18 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.96 g cm −3 |
|||||||||
| Melting point |
|
|||||||||
| boiling point |
221 ° C |
|||||||||
| solubility |
|
|||||||||
| Refractive index |
1.447 (20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
2,5-Hexanediol is a chemical compound from the group of diols . There are three stereoisomers:
- (2 R , 5 R ) -2,5-hexanediol,
- (2 S , 5 S ) -2,5-hexanediol and
- meso -2,5-hexanediol.
Extraction and presentation
(2 R , 5 R ) -hexanediol can be obtained from 2,5-hexanedione .
properties
2,5-Hexanediol is a flammable, hardly inflammable, colorless liquid that is easily soluble in water.
use
2,5-hexanediol can be used for the production of other chemical compounds e.g. B. 2,4-hexadiene can be used. ( 2S , 5S ) -2,5-hexanediol is a precursor of chiral phosphine - catalysts and chiral pharmaceutical intermediates.
Individual evidence
- ↑ a b c d e f g h i j Entry for CAS no. 2935-44-6 in the GESTIS substance database of the IFA , accessed on October 8, 2016(JavaScript required) .
- ↑ a b c d J. Buckingham: Dictionary of Organic Compounds . CRC Press, 1986, ISBN 978-0-412-54090-5 , pp. 3511 ( limited preview in Google Book search).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 298 ( limited preview in Google Book search).
- ↑ Data sheet 2,5-hexanediol, 99% (mixture of isomers) from Sigma-Aldrich , accessed on October 8, 2016 ( PDF ).
- ↑ Jürgen Haberland, Werner Hummel, Thomas Daussmann, Andreas Liese: New Continuous Production Process for Enantiopure (2,5) -Hexanediol. In: Organic Process Research & Development. 6, 2002, p. 458, doi : 10.1021 / op020023t .
- ↑ J. Haberland, A. Kriegesmann, E. Wolfram, W. Hummel, A. Liese: Diastereoselective synthesis of optically active (2R, 5R) -hexanediol. In: Applied Microbiology and Biotechnology. 58, 2002, p. 595, doi : 10.1007 / s00253-002-0936-5 .
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry basics, substance classes, reactions, concepts, molecular structures; 129 tables . Georg Thieme Verlag, 2005, ISBN 978-3-13-541505-5 , p. 86 ( limited preview in Google Book search).
- ↑ M. Bertau, M. Katzberg, W. Hummel, T. Daußmann, J. Stohrer: Biocatalytic synthesis of (S, S) -2,5-hexanediol. In: Chemical Engineer Technology. 78, 2006, p. 1437, doi : 10.1002 / cite.200650458 .
