2,6-di- tert- butylphenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
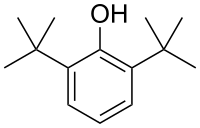
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,6-di-tert-butylphenol | |||||||||||||||
| Molecular formula | C 14 H 22 O | |||||||||||||||
| Brief description |
yellowish solid with a phenolic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 206.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.914 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
34-37 ° C |
|||||||||||||||
| boiling point |
253 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (3 mg l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data |
10,000 mg kg −1 ( LD 50 , rabbit , transdermal ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,6-Di- tert- butylphenol is an aromatic compound . It is a derivative of phenol with two tert-butyl groups .
Extraction and presentation
2,6-di- tert -butylphenol can be prepared by the reaction of phenol with isobutylene and an aluminum phenoxide - catalyst are prepared.
use
2,6-Di- tert -butylphenol is widely used as an antioxidant in fuels, lubricants and polymers. It is also used as a synthetic intermediate product for the production of higher molecular weight phenolic antioxidants.
Individual evidence
- ↑ a b c d e f g h i Entry on 2,6-di-tert-butylphenol in the GESTIS substance database of the IFA , accessed on August 6, 2017(JavaScript required) .
- ↑ Helmut Fiege, Heinz-Werner Voges, Toshikazu Hamamoto, Sumio Umemura, Tadao Iwata, Hisaya Miki, Yasuhiro Fujita, Hans-Josef Buysch, Dorothea Garbe, Wilfried Paulus: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag & Co. KGaA, 2000, ISBN 978-3-527-30673-2 , Phenol Derivatives.
- ↑ OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for phenol, 2,6-bis (1,1-dimethylethyl) - , accessed on July 4, 2016.

