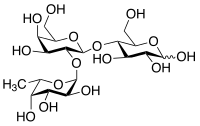
2'-fucosyl lactose
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | 2'-fucosyl lactose | ||||||||||||
| other names |
(2 R , 3 R , 4 R , 5 R ) -4 - [(2 S , 3 R , 4 S , 5 R , 6 R ) -4,5-dihydroxy-6- (hydroxymethyl) -3 - [( 2 S , 3 S , 4 R , 5 S , 6 S ) -3,4,5-trihydroxy-6-methyloxan-2-yl] oxyoxan-2-yl] oxy-2,3,5,6-tetrahydroxy-hexanal |
||||||||||||
| Molecular formula | C 18 H 32 O 15 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 488.44 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| solubility |
easily in water (240 g l −1 at 25 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
2′-Fucosyllactose is a trisaccharide that was found in breast milk in the 1950s . It is therefore a human milk oligosaccharide (HMO). The isolation method most widely used today has been in use since 1972. It also occurs in faeces . It works equivalent to 3′-fucosyllactose .
Extraction and isolation
This substance can be produced in large quantities by E. coli .
The isolation takes place as with other HMO. For this purpose, the fats , proteins and most of the lactose are first split off and, after filtration with a Sephadex gel, paper chromatography is carried out. Sometimes electrophoresis is also required. In the meantime, however, enzymatic approaches are also used for the synthesis of 2'-fucosyllactose.
use
Like other oligosaccharides, 2′-fucosyllactose has the ability to protect against infectious diseases by preventing toxins and pathogens from adhering to the epithelium. It is known that 2′-fucosyllactose protects against, among other things, Campylobacter jejuni , Salmonella enterica serotype Typhimurium and Helicobacter pylori . In the United States , the FDA has approved it for use in baby food at a concentration of 2 g / L or less. It has also been used in assays for glycosyltransferases in blood groups A and B.
Individual evidence
- ↑ a b Entry on 2-Fucosyllactose in the Human Metabolome Database (HMDB) , accessed on May 11, 2018.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ A. Anderson, AS Donald: Improved method for the isolation of 2 ′ fucosyllactose from human milk . In: Journal of Chromatography A . tape 211 , no. 1 , June 26, 1981, pp. 170-174 , PMID 6894927 .
- ↑ Jennewein Biotechnology GmbH: Fucosyllactose - Jennewein Biotechnologie GmbH . In: Jennewein Biotechnologie GmbH . ( jennewein-biotech.de [accessed on May 13, 2018]).
- ↑ Young-Wook Chin, Ji-Yeong Kim, Won-Heong Lee, Jin-Ho Seo: Enhanced production of 2′-fucosyllactose in engineered Escherichia coli BL21star (DE3) by modulation of lactose metabolism and fucosyltransferase . In: Journal of Biotechnology . tape 210 , September 2015, p. 107–115 , doi : 10.1016 / j.jbiotec.2015.06.431 .
- ↑ Akira Kobata: [24] Isolation of oligosaccharides from human milk . In: Methods in Enzymology . Elsevier, 1972, ISBN 978-0-12-181891-3 , pp. 262-271 , doi : 10.1016 / 0076-6879 (72) 28026-0 .
- ↑ Christoph Albermann, Wolfgang Piepersberg, Udo F Wehmeier: Synthesis of the milk oligosaccharide 2′-fucosyllactose using recombinant bacterial enzymes . In: Carbohydrate Research . tape 334 , no. 2 , August 2001, p. 97-103 , doi : 10.1016 / s0008-6215 (01) 00177-x .
- ^ A b Won-Heong Lee, Panchalee Pathanibul, Josh Quarterman, Jung-Hyun Jo, Nam Soo Han: Whole cell biosynthesis of a functional oligosaccharide, 2′-fucosyllactose, using engineered Escherichia coli . In: Microbial Cell Factories . tape 11 , April 30, 2012, p. 48 , doi : 10.1186 / 1475-2859-11-48 , PMID 22545760 , PMC 3442965 (free full text).
- ↑ D TAYLOR, D RASKO, R SHERBURNE, C HO, L JEWELL: Lack of correlation between Lewis antigen expression by and gastric epithelial cells in infected patients . In: Gastroenterology . tape 115 , no. 5 , November 1998, pp. 1113-1122 , doi : 10.1016 / s0016-5085 (98) 70082-4 .
- ↑ GRAS Notices. Retrieved May 11, 2018 .
- ↑ E. Dejana, A. Bonaccorsi, G. de Gaetano: Acetylsalicylic acid and the cardiovascular effects of ADP in the rat . In: Haemostasis . tape 7 , no. 5 , 1978, p. 294-297 , doi : 10.1159 / 000214271 , PMID 689492 .