3,5-dinitrobenzoyl chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
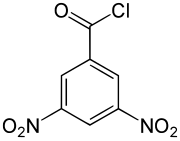
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3,5-dinitrobenzoyl chloride | |||||||||||||||
| Molecular formula | C 7 H 3 ClN 2 O 5 | |||||||||||||||
| Brief description |
yellow needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 230.56 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
68-69 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The 3,5-dinitrobenzoyl chloride is a yellowish-crystalline solid with a melting point of 68-69 ° C. It is the acid chloride of 3,5-dinitrobenzoic acid and is mainly used in the analysis of organic substances.
presentation
3,5-Dinitrobenzoyl chloride is obtained from 3,5-Dinitrobenzoic acid by reaction with phosphorus pentachloride (PCl 5 ). It is also accessible by reaction with phosphorus trichloride (PCl 3 ) or thionyl chloride (SOCl 2 ).
use
The 3,5-dinitrobenzoyl chloride is mainly used in the analysis of organic substances by derivatization , especially of alcohols and amines. It is used in cases when the substance in question is more sensitive and a direct reaction with 3,5-dinitrobenzoic acid is not possible. As a rule, the reaction takes place in pyridine in order to bind the hydrogen chloride released immediately. In this way z. B. derivatives of amino acids also accessible.
 Detection of D, L-alanine by reaction with 3,5-dinitrobenzoyl chloride:
Detection of D, L-alanine by reaction with 3,5-dinitrobenzoyl chloride:
The product melts at 177 ° C.
literature
- Jiří Gasparić, Jiří Borecký: Identification of organic compounds: XLI. Message. Paper chromatographic separation and identification of alcohols, glycols, polyethylene glycols, phenols, mercaptans and amines in the form of their 3,5-dinitrobenzoyl derivatives ; in: Journal of Chromatography A , 1961 , 5 , pp. 466-499 ( doi: 10.1016 / S0021-9673 (01) 92890-0 ).
- WT Robinson, RH Cundiff, PC Markunas: Rapid Determination of Organic Hydroxyl Groups with 3,5-Dinitrobenzoyl Chloride ; in: Anal. Chem. , 1961 , 33 (8), pp. 1030-1034 ( doi: 10.1021 / ac60176a050 ).
See also
Individual evidence
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-214.
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 , p. 216.
- ↑ a b c d data sheet 3,5-Dinitrobenzoyl chloride from Sigma-Aldrich , accessed on March 12, 2017 ( PDF ).
- ↑ a b B. C. Saunders, GJ Stacey, IGE Wilding: The Preparation of 3: 5-Dinitrobenzoic Acid and 3: 5-Dinitrobenzoyl Chloride - Observations on the Acylation of Amino-acids by means of 3: 5-Dinitrobenzoyl Chloride and certain other Acid Chlorides : in: Biochem. J. , 1942 , 36 (3-4), pp. 368-375 ( PMC 1265703 (free full text, PDF), PMID 16747534 ).
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 440.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 424.
- ↑ Bernard Charles Saunders: The Identification of Amino acids by means of 3: 5-Dinitrobenzoyl Chloride ; in: Biochem. J. , 1934 , 28 (2), pp. 580-586 ( PMC 1253231 (free full text, PDF), PMID 16745421 ).
Web links
- Entry to 3,5-dinitrobenzoyl chloride . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD

