3-chloropentane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
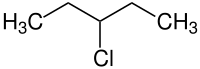
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-chloropentane | |||||||||||||||
| Molecular formula | C 5 H 11 Cl | |||||||||||||||
| Brief description |
colorless to light brown liquid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 106.60 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.87 g cm −3 |
|||||||||||||||
| Melting point |
−105 ° C |
|||||||||||||||
| boiling point |
98 ° C |
|||||||||||||||
| Vapor pressure |
27 hPa (39 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4082 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-chloropentane is a chemical compound of chlorine from the group of aliphatic , saturated halogenated hydrocarbons .
Extraction and presentation
3-chloropentane can be obtained by reacting 3-pentanol with thionyl chloride .
properties
3-chloropentane is a highly flammable, colorless to light brown liquid with an aromatic odor that is very sparingly soluble in water.
safety instructions
The vapors of 3-chloropentane can form an explosive mixture with air ( flash point approx. −1 ° C).
Individual evidence
- ↑ a b c d e f g h i j Entry on 3-chloropentane in the GESTIS substance database of the IFA , accessed on October 26, 2018(JavaScript required) .
- ↑ a b David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 0-8493-0595-0 , pp. 120 ( limited preview in Google Book search).
- ↑ Entry on 3-chloropentane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on October 28, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Richard Lee Jacobs, A Study of the Alkylation of Hydratroponitrile with Amyl Halides . Michigan State College. Department of Chemistry, 1955, OCLC 24559025 , pp. 14 (English, limited preview in Google Book Search).

