Achilles
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
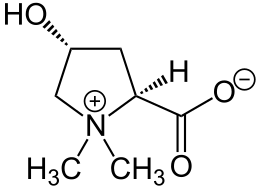
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Achilles | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 13 NO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 159.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.0765 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
254-256 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Achillein (also: L -4-Hydroxystachydrin , L -Betonicin ) is a plant component from the group of pyrrolidine alkaloids . The compound - in the form of its carboxylate - is one of the betaines . Chemically, Achillein is an N , N -dimethylated derivative of the proteinogenic amino acid L - hydroxyproline .
Achilles has anti-inflammatory properties.
Occurrence
Achilles was first found in the medicinal ziest Stachys officinalis , also betonia ; this led to the name "Betonicin". It is also found in various species of the yarrow ( Achillea ) and in the beach bean Canavalia maritima .
Individual evidence
- ^ Carl L. Yaws: Thermophysical properties of chemicals and hydrocarbons. William Andrew, 2008, ISBN 978-0-8155-1596-8 , p. 233.
- ↑ a b Jiaju Zhou, Guirong Xie, Xinjian Yan: Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications. Volume 1: Isolated Compounds A-C. Springer, 2011, ISBN 978-3-642-16734-8 , p. 273.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ E. Nuremberg, P. Surmann: Hager's handbook of pharmaceutical practice. Volume 2, 5th edition. Birkhäuser, 1991, p. 49.
