Acid dyes
The acid dyes , also known as anionic dyes , are a class of dyes that includes compounds with different chromophores . The common structural element of these dyes are solubilizing, anionic substituents . The main application of the acid dyes is the dyeing of wool , polyamide and silk .
Chemical properties
By far the largest number of representatives of the acid dyes belong to the azo dyes . The most common hydrophilic substituent is the sulfonic acid residue - this can easily be introduced into the molecule and is completely dissociated in the pH range (pH 2-6) that is usual in the dyeing process .
The protein fibers of wool and silk contain amino and carboxyl groups , which are present as zwitterions in aqueous solution . In the acid dye bath, on the other hand, the carboxyl groups are not dissociated, while the amino groups are present as ammonium cations. Counterions (chloride, hydrogen sulfate, acetate, formate, etc.) are adsorbed to balance the charge. During the actual dyeing process, these small counterions are then exchanged for dye anions. The dye is thus absorbed from the dye liquor onto the fiber.
Depending on the size of the molecule, acid dyes can be divided into the following groups:
- Rather small dye molecules are characterized by good migratory capacity and essentially form salt-like bonds with the functional groups of the fiber. Very uniform two-dimensional dyeings are therefore obtained (levelness). However, they show a rather poor wash-out behavior and are preferably used in large quantities for coloring cheap articles.
- In the case of products with larger dye molecules, the salt bond only plays a secondary role and the bond to the fiber is essentially based on adsorption forces between the hydrophobic part of the dye molecule and the fiber. This group of acid dyes is characterized by good wet fastness properties. In many cases, however, this advantage is achieved at the expense of the levelness of the dyeings.
Examples
- CI Acid Black 1 gives a blue-black shade on wool with very good light fastness , but moderate wet fastness.
- CI Acid Yellow 36 is one of the oldest acid dyes, which is still used for inexpensive wool dyeing and for some special applications ( leather and paper ).
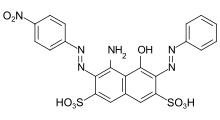
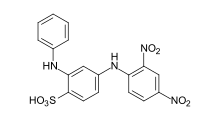
- CI Acid Blue 117 and CI Acid Orange 19 are examples of acid dyes that can be used to achieve very level dyeings with good wet fastness properties.
- CI Acid Blue 25 and CI Acid Orange 3 are examples of acid dyes that do not belong to the azo dyes. CI Acid Blue 25 belongs to the group of anthraquinone dyes and CI Acid Orange 3 is a representative of the nitro dyes .
Note: All structures are given as free acid. The substance information relates to the sodium salts
literature
- Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 276 ff . ( limited preview in Google Book search).
Individual evidence
- ↑ Entry on acid dyes. In: Römpp Online . Georg Thieme Verlag, accessed on January 17, 2019.
- ↑ Paul Rys, Heinrich Zollinger: Guide to dye chemistry . Verlag Chemie, Weinheim 1970, p. 59 ff .
- ↑ External identifiers or database links for Acid Blue 117 : CAS number: 10169-12-7, EC number: 600-233-3, ECHA InfoCard: 100.110.925 , PubChem : 275068951 , Wikidata : Q73275719 .
- ↑ External identifiers or database links to Acid Orange 19 : CAS number: 3058-98-8, EC number: 221-298-0, ECHA InfoCard: 100.019.363 , PubChem : 135601373 , Wikidata : Q72436219 .
- ↑ External identifiers or database links to Acid Orange 3 : CAS number: 6373-74-6, EC number: 228-921-5, ECHA InfoCard: 100.026.292 , PubChem : 5284435 , ChemSpider : 4447503 , Wikidata : Q3621274 .