Aluminum titanate
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
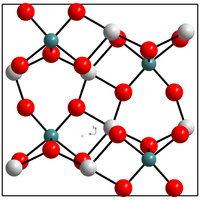
|
||||||||||||||||
| __ Ti 4+ __ Al 3+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Aluminum titanate | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | Al 2 TiO 5 | |||||||||||||||
| Brief description |
White, creamy yellow or gray solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 181.83 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.68 g cm −3 |
|||||||||||||||
| Melting point |
1894 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Aluminum titanate (or Tialit , ATI for short ) is an oxidic compound of aluminum and titanium from the group of titanates , which is important for the ceramics industry due to its special thermal properties, in particular low thermal expansion .
Extraction and presentation
Aluminum titanate can be made in several ways, for example:
- Production of a stoichiometric mixture of fine-grain corundum and rutile, for example in a pressed tablet and sintering
- Solution of titanyl sulphate (TiOSO 4 ) and aluminum sulphate (Al 2 (SO 4 ) 3 ) in distilled water, drying to a powder and subsequent sintering.
By adding various additives (e.g. MgO), aluminum titanate can be stabilized, that is, the decomposition into corundum and rutile in the temperature range (900–1280) ° C is avoided.
properties
Physical Properties
Aluminum titanate has a pseudobrookite structure with an orthorhombic base-face-centered unit cell . The space group is Cmcm (No. 63) .
Along the crystallographic axes there is a clear anisotropy in the thermal expansion, the mean thermal expansion is very low (about 5 · 10 −6 K −1 at 1000 ° C or, according to another source, 0.8 · 10 −6 K −1 , but without specifying the temperature). The coefficient of thermal expansion along the c-axis is negative.
The thermal conductivity is around 2 W / (m K) for aluminum titanate-based ceramics.
Chemical properties
Unstabilized aluminum titanate breaks down into corundum and rutile at higher temperatures. In the presence of silica , aluminum titanate can react to form mullite , corundum and rutile.
use
Due to its good thermal shock resistance and low thermal conductivity, aluminum titanate is used in the refractory industry. The strong anisotropy in the thermal expansion creates microcracks in the structure of titanium-based ceramics, which can further increase the resistance to temperature changes .
Individual evidence
- ^ Eberhard Roos, Karl Maile: Materials science for engineers: Fundamentals, application, testing . Gabler Wissenschaftsverlage 2011. ISBN 978-3-642-17463-6 , p. 307 ( limited preview in Google book search).
- ^ A b c H. Holleck: Material Selection for Hard Coatings . In: J. Vac. Sci. and Tech. A. 1986, 4, 6, pp. 2661-2669.
- ↑ a b Data sheet Aluminum titanate from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ a b J. Zabicki, G. Kimmel, J. Yaaran, L. Zevin: Thermal Anisotropy of Tialite (Al 2 TiO 5 ) by powder XRD. In: NanoStructured Materials. 1995, 6, pp. 675-678.
- ↑ M. Nagano, S. Nagashima, H. Maeda, A. Kato: Sintering behavior of Al 2 TiO 5 base ceramics and their thermal properties . In: Ceramics International. , 1999, 25, pp. 681-687.
- ↑ a b Y. Ohya, K. Hamano, Z. Nakagawa: Effects of some additives on microstructure and bending strength of aluminum titanate ceramic. In: Yogyo -kyokai-shi. 1986, 94, pp. 665-670.
- ^ AE Austin and CM Schwartz: The crystal structure of aluminum titanate . In: Acta Cryst. , 1953, 6, pp. 812-813, doi : 10.1107 / S0365110X53002374 .
- ^ A b R. J. Brook (Ed.), RW Cahn (Ex. Ed.), MB Bever (Sen. Adv. Ed.): Concise Encyclopedia of Advanced Ceramic Materials. 1st edition, Pergamon Press, 1991.
