Ammonium paratungstate
| Crystal structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
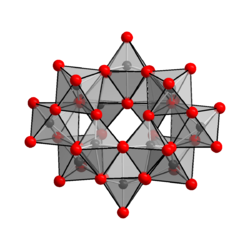
| Anion [H 2 W 12 O 42 ] 10− in the crystal structure of ammonium paratungstate __ W 6+ __ O 2− |
|||||||||||||
| General | |||||||||||||
| Surname | Ammonium paratungstate | ||||||||||||
| other names |
|
||||||||||||
| Ratio formula | (NH 4 ) 10 (H 2 W 12 O 42 ) • 4 H 2 O | ||||||||||||
| Brief description |
colorless, crystalline powder |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 3132.5 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
2.3 g cm −3 |
||||||||||||
| boiling point |
Decomposes above 200 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Ammonium paratungstate (APW or APT for ammonium paratungstate ) is a colorless, crystalline tungsten salt with the chemical formula (NH 4 ) 10 (H 2 W 12 O 42 ) · 4 H 2 O.
Manufacturing
Ammonium paratungstate is an intermediate product in the processing of ores containing tungsten and is obtained through the precipitation of tungstate ions with ammonium ions. Below 50 ° C the undecahydrate forms, while at temperatures above 50 ° C the “pentahydrate” precipitates. The former crystallizes triclinic in the form of platelets or prisms, while the latter forms pseudo-orthorhombic needles.
Tungsten-containing ores such as scheelite (CaWO 4 ) or wolframite ((Mn, Fe) WO 4 ) are initially enriched to concentrations of 10 to 75% by grinding and flotation . The enriched ore is then calcined in an oxidizing atmosphere at 500-600 ° C. in order to remove impurities such as additives from the flotation process. Wolframite ores are then reacted with sodium hydroxide solution , Scheelite ores with a sodium carbonate solution, with sodium tungstate Na 2 WO 4 being formed, which is then purified by a series of reprecipitations. The ammonium paratungstate is obtained from the purified solution obtained by liquid-liquid extraction or ion exchange . Tungsten-containing scrap such as hard metal scrap can also be converted into sodium tungstate after oxidation and then into ammonium tungstate.
Ammonium paratungstate is the globally common form of trade for raw materials containing tungsten and is usually traded in MTUs (metric ton units), with one MTU containing 10 kg of WO 3 .
structure
In more recent literature the anion in ammonium paratungstate is described as [H 2 W 12 O 42 ] 10− , which contains two hydrogen atoms within a cage. The tungsten-oxygen cage, which forms the center of the anion, contains 42 oxygen atoms. Correspondingly, the correct empirical formula of ammonium paratungstate (NH 4 ) 10 [H 2 W 12 O 42 ] · 4 H 2 O and not a "pentahydrate" of the type (NH 4 ) 10 [W 12 O 41 ] as previously described in the literature. · 5H 2 O with five waters of crystallization. The [H 2 W 12 O 42 ] 10− anion is called paratungframation of type B, in contrast to paratungframation, type A, which has the empirical formula [W 7 O 24 ] 6− , corresponding to the paramolybdation. The existence of the paratungstate type A anion has not yet been proven by NMR spectroscopy . Ammonium paratungstate crystallizes monoclinically , space group P 2 1 / n (space group no. 14, position 2) , with the lattice parameters a = 15.08 Å , b = 14.45 Å, c = 11.00 Å and β = 109.4 °.
Reactions
Ammonium paratungstate can be prepared by thermal decomposition at a temperature of 600 ° C in tungsten (VI) oxide WO 3 are converted.
The thermal decomposition of APW takes place in 4 steps:
-
1st step (endothermic reaction between 50 and 190 ° C): In the first step, only the bound crystal water is released but no ammonia . The maximum release is at 159 ° C.
-
2nd step (endothermic reaction between 190 and 250 ° C): In the next step, ammonia is released from the ammonium ions, leaving behind protons.
- 3rd step (endothermic reaction between 250 and 380 ° C.): In the third step there is a further release of 6.3 mol of ammonia together with a corresponding amount of 4.5 mol of water. In this step the - until then largely intact - crystal structure of the anion collapses, making the product X-ray amorphous.
- 4th step (exothermic reaction above 380 ° C): In the last step, the remaining amounts of ammonia and water are released and the remaining tungsten trioxide crystallizes with the release of heat.
use
APW is primarily used as an intermediate product in the processing of ores containing tungsten into tungsten or tungsten compounds. Technically, it is converted into elemental tungsten powder by reduction in a hydrogen atmosphere with the elimination of water vapor, which can be converted into various shapes such as wires or blocks by sintering . By carburizing obtained the foremost in the hard metal industry used tungsten carbide .
Individual evidence
- ↑ a b c d e data sheet ammonium (para) tungstate hydrate from Sigma-Aldrich , accessed on January 23, 2013 ( PDF ).
- ↑ a b M. JG Fait, H.-J. Lunk, M. Feist, M. Schneider, JN Dann, TA Frisk: Thermal decomposition of ammonium paratungstate tetrahydrate under non-reducing conditions: Characterization by thermal analysis, X-ray diffraction and spectroscopic methods . In: Thermochimica Acta . tape 469 , no. 6 , 2008, p. 12–22 , doi : 10.1016 / j.tca.2007.12.007 .
- ↑ Free Online Dictionary: Ammonium paratungstate - What does APT stand for?
- ↑ K. Hempel, M. Saradshow: Solubility and stable crystal hydrates in the ammonium paratungstate – water system. In: Kristall und Technik, Vol. 2, Vol. 3, pp. 437-445, 1967 doi: 10.1002 / crat.19670020316 .
- ^ Sverker Wahlberg: Nanostructured Tungsten Materials by Chemical Methods. Dissertation 2011, urn : nbn: se: kth: diva-42702
- ^ A. Earnshaw, Norman Greenwood: Chemistry of the Elements . 2nd Edition. Butterworth-Heinemann, 1997, ISBN 0-7506-3365-4 , pp. 1012-1014 .
- ↑ H. d'Amour, R. Allmann: The crystal structure of ammonium paratungstate tetrahydrate (NH 4 ) 10 H 2 W 12 O 42 (H 2 O) 4 . In: Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie , 136, 1972, pp. 23-47, doi: 10.1524 / zkri.1972.136.1-2.23 .
- ^ DJ Jones: Practical aspects of sintering tungsten and molybdenum . In: Journal of the Less Common Metals . tape 2 , no. 2-4 , April 1960, pp. 76-85 , doi : 10.1016 / 0022-5088 (60) 90002-3 . Quoted from: JC Bailar Jr. et al .: Comprehensive Inorganic Chemistry. Vol. 3, 1973, p. 744.


![{\ displaystyle \ mathrm {(NH_ {4}) _ {10} [H_ {2} W_ {12} O_ {42}] \ cdot 4H_ {2} O} \ {\ xrightarrow {\ Delta T}} \ \ mathrm {12 \ WO_ {3} +10 \ H_ {2} O + 10 \ NH_ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/de830891154f431bd155564a6ff80b236e71b24a)
![{\ displaystyle \ mathrm {(NH_ {4}) _ {10} [H_ {2} W_ {12} O_ {42}] \ cdot 4H_ {2} O} \ {\ xrightarrow {\ Delta T}} \ mathrm {(NH_ {4}) _ {10} [H_ {2} W_ {12} O_ {42}] + 4 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c614193338796f3471190e10c8843fae8a8a0410)
![{\ displaystyle \ mathrm {(NH_ {4}) _ {10} [H_ {2} W_ {12} O_ {42}]} \ {\ xrightarrow {\ Delta T}} \ mathrm {(NH_ {4}) _ {10-n} H_ {n} [H_ {2} W_ {12} O_ {42}] + n \ NH_ {3}} \ quad {\ text {with}} \ quad n = 2 {,} 7 }](https://wikimedia.org/api/rest_v1/media/math/render/svg/a77774b95ca2dde4f6f7c62b2151a75f17850b68)