Ammonium sulfite
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
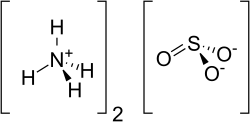
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium sulfite | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | (NH 4 ) 2 SO 3 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 116.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.41 g cm −3 (monohydrate) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium sulfite is an inorganic chemical compound from the group of sulfites .
Extraction and presentation
Ammonium sulphite is formed during flue gas desulphurisation in which sulfur dioxide is washed out of the cooled flue gases after adding ammonia . It is also formed when ammonium thiosulfate decomposes .
properties
Ammonium sulfite is a flammable, white, odorless solid that is easily soluble in water. It decomposes when heated above 60–70 ° C.
use
Ammonium sulphite is used for the production of the food color ammonium sulphite color ( E150d ).
Individual evidence
- ↑ a b c d e f g Entry on ammonium sulfite in the GESTIS material database of the IFA , accessed on July 29, 2014(JavaScript required) .
- ↑ Entry on AMMONIUM SULFITE in the Hazardous Substances Data Bank , accessed on July 29, 2014.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 656.
- ↑ Werner Baltes, Reinhard Matissek: Food chemistry . Springer-Verlag, 2011, p. 258 ( limited preview in Google Book search).
