Anthraquinone dyes
Anthraquinone dyes are an extensive group of dyes , with anthraquinone as a common structural element. Anthraquinone itself is colorless - due to the introduction of electron donor groups, such as. B. hydroxyl or amino groups in the 1-, 4-, 5- or 8-position gives red to blue dyes. Anthraquinone dyes are structurally related to the indigoid dyes and, together with them, are classified in the group of carbonyl dyes .
Representatives of this group of dyes can be found both in natural dyes and in synthetic dyes. Anthraquinone dyes are found in the stain and vat dyes , but also in the reactive and disperse dyes . They are characterized by very good lightfastness .
Natural anthraquinone dyes
One of the most important anthraquinone dyes of plant origin is alizarin , which is obtained from madder ( Rubia tinctorum ). Alizarin is the namesake for a number of structurally related dyes, the alizarin dyes (sometimes used synonymously for the anthraquinone dyes). It was the first natural dye for which an industrial synthesis was developed as early as 1869.
Among the anthraquinone dyes are extracted from scale insects insect dyes carminic , kermesic and Laccainsäuren . The color carmine with the main component carminic acid is used, for example, as an approved food color E 120.
Synthetic anthraquinone dyes
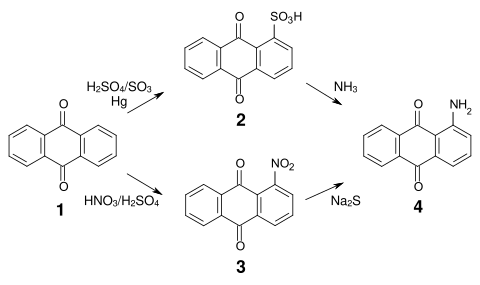
The synthesis of most anthraquinone dyes starts from anthraquinone sulfonic acid ( 2 ) or nitroanthraquinone ( 3 ), which is obtained by sulfonation or nitration of anthraquinone ( 1 ).
The sulfonation in the α-position is reversible and both the sulfonic acid groups and the nitro groups can be exchanged relatively easily for amino, alkylamino, hydroxy and alkoxy groups . Aminoanthraquinone ( 4 ) can be obtained by reacting anthraquinone sulfonic acid with ammonia or by reducing nitroanthraquinone.
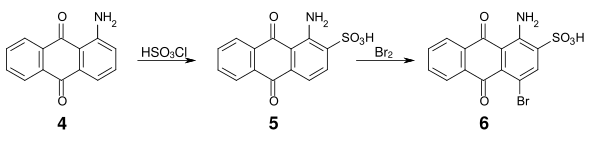
An important intermediate for many acidic anthraquinone dyes is bromamic acid ( 1-amino-4-bromoanthraquinone-2-sulfonic acid ) ( 6 ), which can be obtained from 1-aminoanthraquinone ( 4 ) via sulfonation with chlorosulfonic acid and subsequent bromination .
By replacing the bromine substituent with an aliphatic or aromatic amine, brilliant blue dyes are obtained. For example, bromamic acid can be condensed with 3- (2-hydroxyethylsulfonyl) aniline ( 7 ) to form the brilliant blue dye ( 8 ) ( oxysulfone blue ), from which the reactive dye CI Reactive Blue 19 is obtained after esterification with sulfuric acid .
Reactive Blue 19 is one of the oldest, patented in 1949 and still the most important reactive dyes.
The first synthetic vat dye based on anthraquinone was indanthrone ( CI Vat Blue 4 ) - the synthesis of which was developed in 1901 by René Bohn :
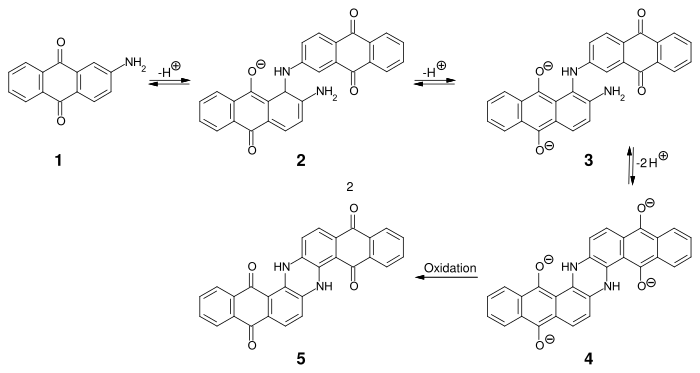
By dimerizing 2-aminoanthraquinone ( 1 ) under strongly alkaline conditions at 220–235 ° C, intermediate 3 is obtained in two steps , which is intramolecularly cyclized and oxidized to indanthrone 5 .
Individual evidence
- ↑ Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 35 ff . ( limited preview in Google Book search).
- ^ Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 255 ff . ( limited preview in Google Book search).
- ↑ Entry on anthraquinone dyes. In: Römpp Online . Georg Thieme Verlag, accessed on December 14, 2018.
- ↑ Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 200 ff . ( limited preview in Google Book search).
- ^ Heinz-Gerhard Franck, Jürgen W. Stadelhofer: Industrial Aromatic Chemistry: Raw Materials · Processes · Products . Springer Verlag, Berlin, Heidelberg 1978, ISBN 978-3-662-07876-1 , p. 365 ff . ( limited preview in Google Book search).
- ↑ Patent DE965902 : Process for fixing water- soluble organic compounds on substrates with a fibrous structure. Registered on July 19, 1949 , published on September 19, 1957 , applicant: Hoechst AG, inventor: Johannes Heyna, Willy Schumacher.
- ↑ Patent DE4422160 : Process for the production of CI Reactive Blue 19. Applied on June 24, 1994 , published on April 1, 1996 , applicant: Hoechst AG, inventor: Andreas Von Der Eltz.
- ^ Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 289 ( limited preview in Google Book search).