1-aminoanthraquinone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-aminoanthraquinone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 9 NO 2 | |||||||||||||||
| Brief description |
dark brown solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 223.23 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
253-255 ° C |
|||||||||||||||
| boiling point |
> 300 ° C |
|||||||||||||||
| Vapor pressure |
1.2 · 10 −4 Pa (100 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-Aminoanthraquinone is a chemical compound from the group of anthraquinone derivatives .
Extraction and presentation
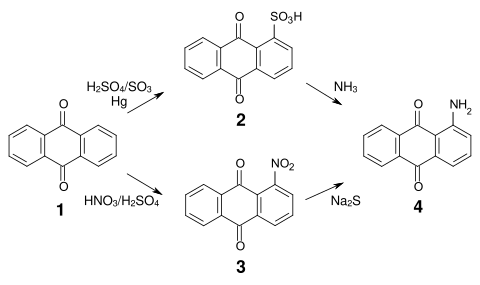
The most important production methods for 1-aminoanthraquinone are the reaction of anthraquinone sulfonic acid ( 2 ) with ammonia, or the reduction of 1-nitroanthraquinone ( 3 ), which is accessible by nitration of anthraquinone ( 1 ).
The reduction of nitroanthraquinone is carried out , for example, with sodium sulfide or sodium hydrogen sulfide . As an alternative to reduction, it is also possible to replace the nitro group with the amino group , this reaction being mainly used in the preparation of secondary amines.
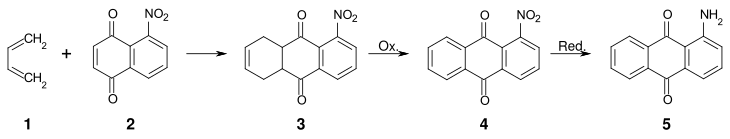
An alternative method for the preparation of 1-aminoanthraquinone by building up the anthraquinone skeleton starts from 5-nitronaphthoquinone .
By reacting 1,3-butadiene ( 1 ) with 5-nitronaphthoquinone ( 2 ) in the sense of a Diels-Alder reaction , 5-nitro-1,4,4a, 9a-tetrahydroanthraquinone ( 3 ) is obtained. Oxidation converts 3 into 1-nitroanthraquinone ( 4 ), which is then reduced to 1-aminoanthraquinone ( 5 ).
properties
1-Aminoanthraquinone is a flammable, difficult to ignite, crystalline, dark brown solid that is practically insoluble in water.
use
1-Aminoanthraquinone is used as an intermediate in the manufacture of anthraquinone dyes and drugs.
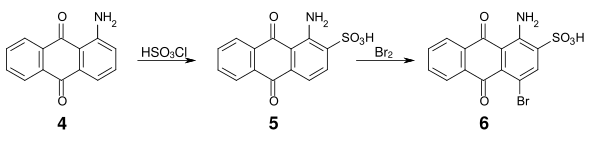
An important intermediate dye product is bromamic acid ( 1-amino-4-bromoanthraquinone-2-sulfonic acid ) ( 6 ), which can be obtained from 1-aminoanthraquinone ( 4 ) via sulfonation with chlorosulfonic acid or oleum and subsequent bromination .
Web links
- Professional association raw materials and chemical industry: TOXICOLOGICAL REVIEWS in long version (05/2002) 1-Aminoanthraquinone , accessed on May 16, 2019
Individual evidence
- ↑ a b c d e f g Entry on 1-aminoanthraquinone in the GESTIS substance database of the IFA , accessed on May 16, 2019(JavaScript required) .
- ↑ a b c d e OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Anthraquinone, 1-amino- , accessed on May 16, 2019.
- ↑ Data sheet 1-Aminoanthraquinone, 97% from AlfaAesar, accessed on May 16, 2019 ( PDF )(JavaScript required) .
- ↑ Entry on Aminoanthraquinones. In: Römpp Online . Georg Thieme Verlag, accessed on May 21, 2019.
- ↑ Willy Herbst, Klaus Hunger: Industrial Organic Pigments Production, Properties, Applications . John Wiley & Sons, 2006, ISBN 978-3-527-60406-7 , pp. 501 ( limited preview in Google Book search).
- ↑ Hans-Samuel Bien, Klaus Wunderlich: Anthraquinone dyes and intermediates . In: Ullmann's Encyclopedia of Technical Chemistry . 4th edition. tape 7 . Verlag Chemie, Weinheim 1974, ISBN 3-527-20000-2 , pp. 585 ff .
- ↑ Patent application DE2539631 : Process for the production of high-purity 5-nitro-1,4,4A, 9A-tetrahydroanthraquinone. Applied September 5, 1975 , published March 18, 1976 , Applicant: Mitsui Toatsu Chemicals, Inventor: Torisu Yasuyoshi, Kaba Seishichiro, Mukai Ken.
- ↑ EN Abrahart: Dyes and Their Intermediates . 2nd Edition. Edward Arnold (Publishers) Ltd., London 1977, ISBN 0-7131-2580-2 , pp. 41-42 .