Anthron
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Anthron | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 10 O | |||||||||||||||
| Brief description |
light yellow, almost odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 194.23 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
151-156 ° C |
|||||||||||||||
| boiling point |
721 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Anthrone is a tricyclic compound . In biochemistry , this compound is used to determine the total content of carbohydrates, including glycerol, in biological samples. Anthrone is - like anthraquinone - a derivative of anthracene and is considered an active ingredient in herbal medicine .
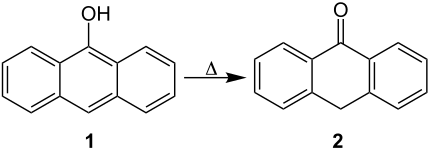
Manufacturing
Rapid heating of anthranol ( 1 ) produces anthrone ( 2 ):
1 can be viewed as the transannular enol form of 2 .
properties
Anthrone dissolves in alkaline solutions when heated ; when acidified, anthranol, the enol form , precipitates .
Web links
Commons : Anthron - collection of images, videos and audio files
Individual evidence
- ↑ a b c d e data sheet Anthron (PDF) from Merck , accessed on December 23, 2012.
- ↑ Data sheet Anthron at Acros, accessed on February 26, 2010.
- ^ Brockhaus ABC chemistry. FA Brockhaus Verlag, Leipzig 1965, p. 93.
- ↑ A. Pons, P. Roca, C. Aguilo, FJ Garcia, M. Alemany, A. Palou: A method for the simultaneous determination of total carbohydrate and glycerol in biological samples with the anthrone reagent. In: J Biochem Biophys Methods . 4 (3-4), 1981, pp. 227-331. PMID 7240650 .
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 336, ISBN 3-342-00280-8 .