Apremilast
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Apremilast | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 22 H 24 N 2 O 7 S | |||||||||||||||||||||
| Brief description |
crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 460.5 g · mol -1 | |||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Apremilast (trade name: Otezla ; manufacturer: Amgen ) is a drug from the group of phosphodiesterase inhibitors that is used in the treatment of moderate to severe chronic plaque psoriasis and psoriatic arthritis . The active ingredient was approved for these indications in 2014 in the USA and on January 15, 2015 in Europe.
Clinical information
Application areas (indications)
Psoriatic arthritis
Apremilast alone or in combination with disease-modifying anti-rheumatic drugs (DMARDs) is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response to previous DMARD therapy or who have not tolerated it.
psoriasis
Apremilast is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who have not responded to other systemic therapy, such as ciclosporin or methotrexate or psoralen in combination with UVA light (PUVA), or for whom such therapy is contraindicated or who have not tolerated it.
Type and duration of application
The normal dosage of apremilast is 60 mg per day in two tablets of 30 mg of active ingredient each (morning and evening). In order to avoid gastrointestinal complaints, tablets of 10 mg or 20 mg active ingredient content should be used for 6 days.
Contraindications (contraindications)
Hypersensitivity to the active ingredient or any of the excipients.
Use during pregnancy and breastfeeding
Apremilast is contraindicated during pregnancy and should not be used during breastfeeding. Pregnancy must be ruled out prior to treatment. Women of childbearing potential must use effective contraception to prevent pregnancy.
The substance is assigned to the Pregnancy Risk Category C by the FDA. It is unknown whether the substance is excreted in breast milk. Metabolites were found in the milk of mice.
Special warnings and precautions for use
People with the following rare diseases should not take the drug:
- Galactose intolerance
- Galactose lactase deficiency
- Glucose-galactose malabsorption
With severely reduced kidney function, the drug must be dosed lower.
In people who are underweight, their body weight should be checked regularly before treatment. Discontinuation of treatment should be considered if weight loss is noticeable.
Drug interactions
Strong inducers of cytochrome P450 enzymes , e.g. B. rifampicin , phenobarbital or phenytoin , lead to a decreased plasma level of apremilast.
Special patient groups
The substance is only approved for the treatment of adult patients.
In persons with severe renal impairment ( creatinine clearance ≤ 30 ml min −1 ) the dose should be adjusted to 30 mg once daily. Careful risk assessment is necessary for those who have or have suffered from depression or who are known to be suicidal.
Adverse effects (side effects)
Common :
- depression
- insomnia
- diarrhea
- nausea
- Vomit
- Upper respiratory tract infections
- Rhinopharyngitis
- Pain in the upper abdomen
Frequency unspecified:
- Hypersensitivity reactions
- Weight loss
- frequent bowel movements
- Reflux esophagitis
- dyspepsia
- decreased appetite
- migraine
- to cough
- Rash
Pharmacological properties
Mechanism of action (pharmacodynamics)
By inhibiting PDE-4, apremilast leads to an increase in the intracellular cAMP level in synoviocytes , which leads to a reduced release of proinflammatory TNF-α .
Absorption and distribution in the body (pharmacokinetics)
absorption
Taken orally, apremilast has a bioavailability of approx. 73%. Plasma level peaks (C max ) are reached after approx. 2.5 hours. Taking it with a meal does not affect the absorption of apremilast.
distribution
Plasma protein binding is approximately 68%. The volume of distribution is 87 L.
Metabolism
After oral ingestion, unchanged apremilast is found in the bloodstream as the main component (45%), followed by an inactive metabolite, which is a glucuronide of O-demethylated apremilast. Apremilast is extensively metabolised in humans and forms up to 23 metabolites, which can be detected in plasma, urine and faeces. The degradation takes place both through CYP-enzymatic oxidation and subsequent glucuronidation as well as through hydrolysis by other enzymes. In vitro it has been shown that apremilast is primarily metabolized by CYP3A4 and to a lesser extent also by CYP1A2 and CYP2A6 .
elimination
The clearance of apremilast is approx. 10 l / h in healthy individuals. The elimination half-life is 6–9 hours. After oral administration of radioactively labeled apremilast, 58% (urine) and 39% (faeces) of the radioactive dose could be recovered. 3% (urine) and 7% (faeces) were unchanged apremilast.
Other Information
Chemical and pharmaceutical information
synthesis
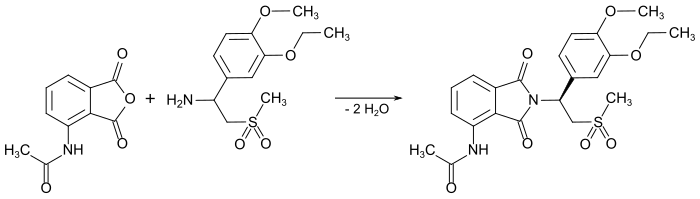
A solution of equal proportions of 1- (3-ethoxy-4-methoxyphenyl) -2-methylsulfonylethylamine and 3-acetamidophthalic anhydride is refluxed in acetic acid for 15 h. The product is formed as a yellow solid with a melting point of 144 ° C. and a yield of 59%.
Analytics
Instrument-based analytics
Apremilast shows the following chemical shifts in 1 H-NMR :
Early benefit assessment
In Germany, since 2011, newly approved drugs with new active ingredients must be subjected to an " early benefit assessment " by the Federal Joint Committee (G-BA) in accordance with Section 35a SGB V if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. The dossier evaluations, on the basis of which the G-BA makes its decisions, are created by the Institute for Quality and Efficiency in Health Care (IQWiG) .
In the early benefit assessment, Apremilast 2015 was treated with a TNF-alpha inhibitor (etanercept or adalimumab or infliximab or golimumab) for active psoriatic arthritis in adults who had inadequately responded to previous DMARD therapy or who did not tolerate it Combination compared with methotrexate. For adults with moderate to severe chronic plaque psoriasis who have not responded to other systemic therapy such as ciclosporin or methotrexate or psoralen in combination with UVA light or for whom such therapy is contraindicated or who could not tolerate it, adalimumab was or Infliximab or ustekinumab were the appropriate comparator therapy. According to the G-BA decision, an additional benefit compared to these appropriate comparator therapies has not been proven for either of the two indications.
Trade names
Otezla
Others
As part of the alliance between Celgene and Bristol-Myers Squibb (Merger), it was announced in June 2019 that Otezla would have to be sold. The drug made over $ 1.5 billion in sales in 2018. In the pharmaceutical industry, one speaks of a blockbuster (ie> 1.0 billion US dollars in sales).
Individual evidence
- ↑ a b c d Cayman Chemical: Apremilast (608141-41-9) , accessed December 21, 2019.
- ↑ Daniel Purich: The inhibitor Index A Desk Reference on Enzyme Inhibitors, Receptor Antagonists, Drugs, Toxins, Poisons, Biologics, and Therapeutic Leads . CRC Press, 2017, ISBN 978-1-351-73067-9 , pp. 1138 ( limited preview in Google Book search).
- ↑ a b c d e f g h i j k l m n o p q Celgene: Specialist information Otezla . January 2016.
- ↑ a b Otezla Prescribing Information . In: Prescribing Information . Celgene Corporation. Retrieved July 5, 2014.
- ↑ AxonMedchem entry on Apremilast . axonmedchem.com. Archived from the original on July 28, 2014. Retrieved July 5, 2014.
- ↑ a b Patent US6011050A : Substituted phenethylsulfones and method of reducing TNFα levels. Published January 4, 2000 , Inventor: George W. Muller, Hon-Wah Man.
- ↑ A15-09 Apremilast - benefit assessment according to Section 35a SGB V (dossier assessment); Accessed March 24, 2020.
- ↑ Benefit assessment procedure for the active ingredient apremilast (plaque psoriasis, psoriatic arthritis); Accessed March 24, 2020.
- ↑ Bristol-Myers Squibb Provides Update on Pending Merger with Celgene , PM BMS, June 24, 2019, accessed July 12, 2019.
- ↑ US Trade Commission takes a closer look at Celgene acquisition , accessed July 12, 2019.