Avanafil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
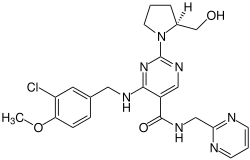
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Avanafil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 23 H 26 ClN 7 O 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 483.951 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Avanafil is a drug from the group of PDE-5 inhibitors that is used in the treatment of erectile dysfunction .
Clinical information
application areas
Avanafil is used to treat erectile dysfunction in adult men. The recommended dose of avanafil is 100 mg, taken about 30 minutes before sexual activity, if needed. Some studies have shown that Avanafil works within 15 minutes and can last for over 6 hours.
Sexual arousal is necessary for PDE-5 inhibitors to work. Depending on the individual effectiveness and tolerance, the dose can be increased to a maximum of 200 mg or reduced to 50 mg. Compared to other PDE-5 inhibitors, avanafil was shown to have a higher selectivity for phosphodiesterase-5 (PDE-5) , which results in better tolerability.
Contraindications
For men with heart disease, the attending physician must consider the potential risk of sexual activity to the heart before prescribing avanafil. The medicine must not be used in patients with certain severe heart or circulation problems, including those who have had a heart attack, stroke, severe arrhythmia (irregular heartbeat) or unstable angina (a type of severe chest pain) in the last six months , Angina during intercourse, heart failure, or high or low blood pressure. Avanafil must also not be used in patients whose liver or kidney function is severely impaired, or who have had vision loss from a disorder in the blood flow to the optic nerve (non-arteritic anterior ischemic optic neuropathy, NAION) that may be caused by this class of drugs.
Drug interactions
Avanafil must not be taken with certain other drugs such as nitrates (a certain type of medicine for angina pectoris ) or the sex drug " Poppers " (also known as "PP" in the MSM scene), as these are also nitrates contain which in combination with avanafil but also in combination with all other PDE-5 inhibitors can lead to a life-threatening drop in blood pressure and subsequently to cardiac arrest. Caution is also advised with the inhibitors of the cytochrome CYP3A4 (from the cytochrome P450 family ), which greatly reduce the metabolism of avanafil in the body and, in addition, lead to a sharp increase in the avanafil level in the blood and, consequently, the occurrence of undesirable side effects can lead or can significantly increase side effects. The inhibitors of cytochrome CYP3A4 include: ketoconazole , ritonavir , cobicistat , atazanavir , clarithromycin , indinavir , itraconazole , nefazodone , nelfinavir , saquinavir and telithromycin (... list not exhaustive). If treatment with the aforementioned drugs or a drug from the group of protease inhibitors ( PI ) (a class of active substances from HIV therapy) is required, it is essential to adjust the dose. The starting dose of 100 mg / 48 hours should not be exceeded in this case.
unwanted effects
Avanafil has no very common (> 1/10) side effects . Common side effects (> 1/100 to <1/10) with avanafil are headache, flushing and nasal congestion. For the full list of all side effects reported with avanafil, see the package leaflet.
Pharmacological properties
Mechanism of action
An erection is the result of the interaction of two endogenous substances: cyclic guanosine monophosphate (cGMP) and phosphodiesterase type 5 (PDE-5) . cGMP relaxes the smooth muscle cells in the erectile tissue of the penis and thus increases the blood flow. This leads to an erection. Phosphodiesterase type 5 (PDE-5), on the other hand, ensures that cGMP is broken down, which leads to the erection subsiding. Avanafil is a highly selective and reversible inhibitor of (cGMP) -specific PDE-5. This means that Avanafil inhibits PDE-5 and thus the cGMP necessary for an erection is not broken down. The concentration of cGMP increases and an erection occurs, which can be sustained long enough to carry out sexual activity satisfactorily. Sexual stimulation is a prerequisite for the development of an erection even when taking avanafil.
Absorption and distribution in the body
Avanafil is characterized by its rapid onset of action. It should be taken about 30 minutes before starting sexual activity. The recommended maximum frequency is once a day. Avanafil can start working within 15 minutes and last longer than 6 hours.
Eligibility for reimbursement
In Germany, the health insurances do not cover the costs for avanafil and patients have to pay for the therapy, just like with other PDE-5 inhibitors. Since January 1, 2004, the Statutory Health Insurance Modernization Act of November 14, 2003 ( Federal Law Gazette I, p. 2190 ) in Section 34 Paragraph 1 Clause 7 and 8 SGB V has excluded medicinal products that are used to improve the quality of life . These include u. a. Medicines that are mainly used to treat erectile dysfunction or to stimulate and increase sexual potency. The cause of the fault is irrelevant. An exception is not provided.
Trade names
Avanafil has been approved in the EU under the name Spedra ( Menarini ) since June 21, 2013 and has been available on prescription since April 2014 in tablet form in the dose strengths 50 mg, 100 mg and 200 mg. The preparation was made ready for approval by Vivus Inc. (proprietary spelling VIVUS) and is marketed in Germany by Berlin-Chemie AG (Menarini) .
The US FDA approved Avanafil at the end of April 2012; the trade name in the USA is Stendra .
literature
- M. Sanford: Avanafil: a review of its use in patients with erectile dysfunction. In: Drugs & aging. Volume 30, Number 10, October 2013, pp. 853-862, doi: 10.1007 / s40266-013-0112-x . PMID 23955441 (Review).
- C. Zhao, SW Kim et al .: Efficacy and safety of avanafil for treating erectile dysfunction: results of a multicentre, randomized, double-blind, placebo-controlled trial. In: BJU international. Volume 110, number 11, December 2012, pp. 1801-1806, doi: 10.1111 / j.1464-410X.2012.11095.x . PMID 22448738 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ LH Belkoff et al .: An open-label, long-term evaluation of the safety, efficacy and tolerability of avanafil in male patients with mild to severe erectile dysfunction. In: Int J Clin Pract. 67, April 2013, pp. 333-341. doi: 10.1111 / ijcp.12065 . PMID 23521325 .
- ^ I. Goldstein, AR McCullough, LA Jones et al.: A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. In: J Sex Med . 9, 2012, pp. 1122-1133. doi: 10.1111 / j.1743-6109.2011.02629.x . PMID 22248153 .
- ^ I. Goldstein, LA Jones, L. Belkoff et al .: Avanafil for the treatment of erectile dysfunction: a multicenter, randomized, double-blind study in men with diabetes mellitus. In: Mayo Clin Proc. 87 (9), August 2012, pp. 843-852. doi: 10.1016 / j.mayocp.2012.06.016 . PMID 22857780 .
- ↑ JP Mulhall, AL Burnett, R. Wang et al.: A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. In: J Urol . 189, 2013, pp. 2229-2236. doi: 10.1016 / j.juro.2012.11.177 . PMID 23219537 .
- ↑ R. Wang et al .: Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: implications for clinical safety and improved tolerability. In: J Sex Med. 9, August 2012, pp. 2122-2129. doi: 10.1111 / j.1743-6109.2012.02822.x . PMID 22759639 .
- ↑ a b c d Summary of the EPAR for the public (German) , website of the European Health Authority (EMA), accessed on February 21, 2014 (PDF; 81 kB).
- ^ Avoxa media group German Pharmacists GmbH: Tadalafil and Vardenafil. In: Pharmaceutical newspaper online. Retrieved July 22, 2017 .
- ↑ a b Erectile Dysfunction - New PDE5 Inhibitor Avanafil. Website of the Deutsche Apotheker Zeitung (DAZ), accessed on February 21, 2014.
- ↑ European public assessment report (EPAR) for Spedra - Avanafil , website of the European Health Authority (EMA), accessed on February 21, 2014.
- ↑ Information for patients ( Memento of September 23, 2015 in the Internet Archive ) Instructions for use Spedra ® (200 mg).
- ↑ Information for patients ( Memento of September 23, 2015 in the Internet Archive ) Instructions for use Spedra ® (50 mg).
- ↑ 1500 sex medicine specialists from over 70 countries met in Istanbul , Bild.de on February 2, 2014, accessed on February 21, 2014.
- ↑ VIVUS Announces Avanafil Partnership With Menarini ( Memento of December 8, 2015 in the Internet Archive ), press release of Vivus Inc. of July 9, 2013, accessed on February 21, 2014.