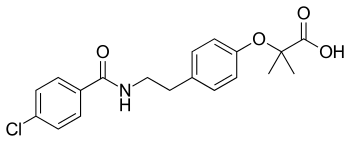
Bezafibrate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Bezafibrate | |||||||||||||||||||||
| other names |
2- (4- {2 - [(4-chlorobenzoyl) amino] ethyl} phenoxy) -2-methylpropionic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 19 H 20 ClNO 4 | |||||||||||||||||||||
| Brief description |
white crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 361.82 g · mol -1 | |||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Bezafibrate is an active ingredient from the group of fibrates . It lowers the triglycerides and the low density lipoproteins and increases the high density lipoproteins . Boehringer Mannheim introduced the active ingredient in 1977 (trade name: Cedur ).
Like other fibrates, bezafibrate acts as an agonist of the peroxisome proliferator-activated receptor PPARα . According to some studies, it also affects the activity of PPARγ and PPARδ.
Common side effects include loss of appetite. In rare cases, myopathy , acute renal failure , dizziness and cholestasis have been reported. In very rare cases, rhabdomyolysis , gallstones and changes in the blood count have also been reported.
A newer treatment indication, for which the drug has not yet been officially approved, is emerging in the treatment of primary biliary cholangitis (PBC). It is considerably cheaper and seems to be as effective as the newly developed obeticholic acid.
literature
- I. Goldenberg, M. Benderly, U. Goldbourt: Update on the use of fibrates: focus on bezafibrate. In: Vascular health and risk management. Volume 4, Number 1, 2008, pp. 131-141, PMID 18629356 . PMC 2464751 (free full text) (review).
Web links
- Bezafibrate . In: drugs from A-Z .
Individual evidence
- ↑ a b c d e Bezafibrate data sheet at Sigma-Aldrich , accessed on February 23, 2013 ( PDF ).
- ↑ Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study . PMID 10880410
- ↑ Christophe Corpechot, Olivier Chazouillères, Alexandra Rousseau, Antonia Le Gruyer, François Habersetzer: A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis . In: New England Journal of Medicine . June 6, 2018, doi : 10.1056 / NEJMoa1714519 ( nejm.org [accessed December 10, 2018]).