Butallylonal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
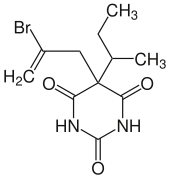
|
||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Butallylonal | |||||||||||||||
| other names |
( RS ) -5- (2-bromoallyl) -5- sec -butylbarbituric acid |
|||||||||||||||
| Molecular formula | C 11 H 15 BrN 2 O 3 | |||||||||||||||
| Brief description |
Crystals with a slightly bitter taste |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 303,15 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
130-133 ° C |
|||||||||||||||
| solubility |
soluble in ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Butallylonal is a barbiturate . From a chemical point of view, it is a racemate made up of two barbituric acid enantiomers. It was developed in the 1960s and mainly used in veterinary medicine, but currently there are no more preparations based on butallylonal; it has been completely replaced by pentobarbital .
Stereochemistry
Butallylonal has a stereogenic center in the side chain of the heterocyclic ring . Butallylonal is chiral , there is an ( R ) form and an ( S ) form, which are present in a ratio of 1: 1, i.e. represent a racemate .
Legal status
Butallylonal is a marketable and prescription narcotic drug in the Federal Republic of Germany due to its listing in Appendix 3 BtMG. Handling without permission or prescription is generally punishable. Further information can be found in the main article Narcotics Law in Germany .
Internationally, butallylonal falls under the Convention on Psychotropic Substances .
Individual evidence
- ↑ a b F.v. Bruchhausen, Siegfried Ebel, AW Frahm, E. Hackenthal: Hager's handbook of pharmaceutical practice substances AD . Springer-Verlag, 2013, ISBN 978-3-642-57995-0 , pp. 516 ( limited preview in Google Book search).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2012, ISBN 978-1-4398-8049-4 , pp. 60 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Thomas Geschwinde: Drugs market forms and modes of action . Springer-Verlag, 2013, ISBN 978-3-662-09677-2 , pp. 531 ( limited preview in Google Book search).