Coproporphyrinogen oxidase
| Coproporphyrinogen oxidase | ||
|---|---|---|

|
||
| Properties of human protein | ||
| Mass / length primary structure | 344 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene names | CPOX CPO; CPX; HCP | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.3.3.3 , oxidoreductase | |
| Response type | Oxidative decarboxylation | |
| Substrate | Coproporphyrinogen III + O 2 + 2 H + | |
| Products | Protoporphyrinogen IX + 2 CO 2 + 2 H 2 O | |
| Occurrence | ||
| Homology family | CPOX | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 1371 | 12892 |
| Ensemble | ENSG00000080819 | ENSMUSG00000022742 |
| UniProt | P36551 | P36552 |
| Refseq (mRNA) | NM_000097 | NM_007757 |
| Refseq (protein) | NP_000088 | NP_031783 |
| Gene locus | Chr 3: 98.52 - 98.59 Mb | Chr 16: 58.67 - 58.68 Mb |
| PubMed search | 1371 |
12892
|
Coproporphyrinogen oxidase (CPOX) is the enzyme that the oxidation of coproporphyrinogen III to protoporphyrinogen IX catalyses a partial reaction in the biosynthesis of Porphyrins , which takes place in all living beings. In eukaryotes , the substrate is first transported from the cytosol into the space between the inner and outer membrane of the mitochondria ; the transporter involved is still unknown. When people can mutations on CPOX - gene to CPOX deficiency and thus hereditary coproporphyria lead.
Catalyzed reaction
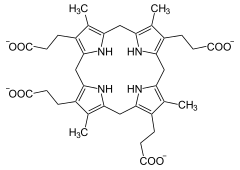 + O 2 + 2 H + ⇒
+ O 2 + 2 H + ⇒
⇒  + 2 CO 2 + 2 H 2 O
+ 2 CO 2 + 2 H 2 O
Coproporphyrinogen III is oxidized to protoporphyrinogen IX, with simultaneous splitting off of carbon dioxide. Two oxidative decarboxylations take place one after the other. The enzyme does not require metal ions as a cofactor in humans.
Individual evidence
- ↑ UniProt P36551
- ↑ Jassal, D'Eustachio / reactome: Translocation of coproporphyrinogen III from the cytosol to the mitochondrial intermembrane space
- ↑ Martásek P, Camadro JM, Raman CS, et al. : Human coproporphyrinogen oxidase. Biochemical characterization of recombinant normal and R231W mutated enzymes expressed in E. coli as soluble, catalytically active homodimers . In: Cell. Mol. Biol. (Noisy-le-grand) . 43, No. 1, February 1997, pp. 47-58. PMID 9074788 .
- ↑ Medlock AE, Dailey HA: Human coproporphyrinogen oxidase is not a metalloprotein . In: J. Biol. Chem. . 271, No. 51, December 1996, pp. 32507-10. PMID 8955072 .
- ↑ Stephenson JR, Stacey JA, Morgenthaler JB, Friesen JA, Lash TD, Jones MA: Role of aspartate 400, arginine 262, and arginine 401 in the catalytic mechanism of human coproporphyrinogen oxidase . In: Protein Sci. . 16, No. 3, March 2007, pp. 401-10. doi : 10.1110 / ps.062636907 . PMID 17242372 . PMC 2203308 (free full text).
Web links
- OMA: CPOX homologues
- Jassal, D'Eustachio / reactome: Conversion of coproporphyrinogen III to protoporphyrinogen IX