Cadmium hexafluorosilicate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
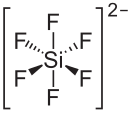
|
|||||||||||||||||||
| Crystal system | |||||||||||||||||||
| Space group |
R 3 (No. 148) |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cadmium hexafluorosilicate | ||||||||||||||||||
| other names |
Cadmium fluorosilicate |
||||||||||||||||||
| Molecular formula | CdSiF 6 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 254.49 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
easily soluble in water (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Cadmium hexafluorosilicate, CdSiF 6 is a chemical compound of cadmium from the group of hexafluorosilicates .
Extraction and presentation
The hexahydrate of cadmium hexafluorosilicate can be obtained by reacting cadmium carbonate with hexafluoridosilicic acid.
properties
Cadmium hexafluorosilicate is a crystalline, colorless solid that is easily soluble in water. The hexahydrate gives off water of crystallization at 170 ° C. It has a crystal structure with the space group R 3 (space group no. 148) .
toxicology
The inhalation or ingestion of the compound is toxic. Cadmium hexafluorosilicate is suspected of causing cancer and organ damage.
literature
- Ganesh Thakur, AL Verma: Raman spectroscopic study of phase transformation in single crystals of cadmium fluorosilicate hexahydrate . John Wiley & Sons Ltd., June 1989, doi : 10.1002 / jrs.1250200605 .
Individual evidence
- ↑ a b c d e Entry on cadmium hexafluorosilicate in the GESTIS substance database of the IFA , accessed on March 16, 2019 (JavaScript required)
- ↑ Entry on cadmium hexafluorosilicate (2−) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 15, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ RJ Meyer: Cadmium system number 33 . Springer-Verlag, 2013, ISBN 978-3-662-11295-3 , pp. 154 ( limited preview in Google Book search).
- ↑ R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 370 ( limited preview in Google Book search).
- ↑ Ganesh Thakur, AL Verma: Raman spectroscopic study of phase transformation in single crystals of cadmium fluorosilicate hexahydrate. In: Journal of Raman Spectroscopy. 20, 1989, p. 367, doi : 10.1002 / jrs.1250200605 .


