Calcium chlorite
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
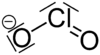
|
||||||||||
| General | ||||||||||
| Surname | Calcium chlorite | |||||||||
| Molecular formula | Ca (ClO 2 ) 2 | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 174.98 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
2.71 g cm −3 |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Calcium chlorite is an inorganic chemical compound of calcium from the group of chlorites .
Extraction and presentation
Calcium chlorite can be produced by reacting chlorous acid with calcium carbonate , whereby the hexahydrate is formed from the solution .
properties
Calcium chlorite is an oxidizing white solid that decomposes when it comes into contact with water, forming calcium hydroxide and chlorine dioxide .
The compound has an orthorhombic crystal structure with the space group Ccce (space group no. 68) and is isotype with strontium chlorite and lead chlorite .
safety instructions
Calcium chlorite itself does not burn, but increases the risk of fire if it comes into contact with flammable substances and can significantly promote an existing fire.
Individual evidence
- ↑ a b c d e Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 77 ( limited preview in Google Book search).
- ↑ a b c Entry on calcium chlorite in the GESTIS substance database of the IFA , accessed on February 27, 2017(JavaScript required) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ The former name of this group of rooms was Ccca .

