Ceftaroline fosamil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
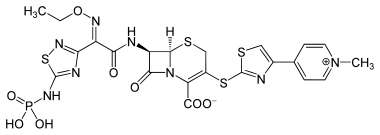
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Ceftaroline fosamil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 22 H 21 N 8 O 8 PS 4 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 684.7 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ceftaroline Fosamil is a medicinal substance with an antibiotic effect .
It is the N- phosphono - prodrug of Ceftarolin, a cephalosporin antibiotic 5th generation with high efficacy against methicillin-resistant staphylococci (MRSA) and other multi-resistant problem bacteria ( VISA , VRSA , PRSP ), and against gram-negative bacteria , but not against ESBL -generating Enterobacteriaceae and non-fermenters.
It was developed by the Japanese pharmaceutical company Takeda and approved by the FDA as Teflaro in October 2010 for the parenteral treatment of adults with community-acquired pneumonia and acute skin and soft tissue infections. In August 2012 the approval for the EU countries followed under the name Zinforo . The active ingredient is used pharmaceutically as the salt of acetic acid , ceftaroline fosamil acetate monohydrate .
literature
- P. Jungmayr: New weapon against problem germs , Deutsche Apothekerzeitung, January 24, 2013.
- Andes, D .; Craig, WA; Antimicrobial Agents and Chemotherapy , 50 (4), 1376-1383, 2006.
- Jaqueline, C .; Caillon, J .; Le Mabecque, V .; Miegeville, A .; Hamel, A .; Bugnon, D .; Yiong Ge, J .; Potel, G .; Antimicrobial Agents and Chemotherapy , 51 (9), 3397-3400, 2007.
- Talbot, GH; Thye, D .; The A.; Ge, Y .; Antimicrobial Agents and Chemotherapy , 51 (10), 3612-3616, 2007.
- Parish, D .; Scheinefeld, N .; Current Opinion in Investigational Drugs , 9 (2), 201-209, 2008.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ FDA Approves Teflaro for Bacterial Infections , FDA News Release, October 29, 2010.
- ↑ Zinforo on the website of the European Medicines Agency.
- ↑ External identifiers or database links for ceftaroline fosamilacetate (1: 1) 1 H 2 O : CAS number: 400827-55-6, EC number: 807-903-6, ECHA InfoCard: 100.235.826 , PubChem : 56841981 , Wikidata : Q72515487 . Molar mass: 762.75 g mol −1 .