Cilofungin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
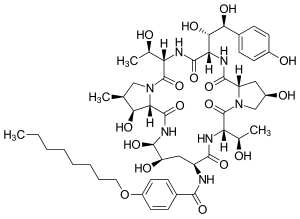
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Cilofungin | ||||||||||||
| other names |
1 - ((4 R , 5 R ) -4,5-dihydroxy- N 2- (4- (octyloxy) benzoyl) -l-ornithine) echinocandin B ( IUPAC ) |
||||||||||||
| Molecular formula | C 49 H 71 N 7 O 17 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 1030.14 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Cilofungin is an antifungal from the echinocandins group . It inhibits the cell wall biosynthesis in some Candida albicans species. Phase II development was discontinued due to the toxicity of the carrier substance (manufacturer: Eli Lilly & Co. ).
pharmacology
Cilofungin inhibits glucan synthase , which is responsible for the formation of beta-1-3- glucan , a major component of most pathogenic fungi . This makes the cell wall unstable. In addition, the mycelium growth is inhibited and the uptake of N -acetylglucosamine in the mycelium is blocked.
literature
Analytics:
- Wood, Miller, Wright, Mc Carthy, Traft, Pomponi, Selitremihoff: Journal of Antibiotics 51, pp. 665-675 (1998).
Synthesis:
- Debono, Abbott, Fukuda, Barnhart, Eillard, Molloy, Michel, Turner, Butler, Hunt: Journal of Antibiotics 42 (3), pp. 389-397 (1998).
Pharmacology:
- Beaulieu, Tang, Yan, Vessels, Radding, Parr: Antimicrobial Agents and Chemotherapy 38 (5), pp. 937-944 (1994).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on cilofungin in the ChemIDplus database of the United States National Library of Medicine (NLM) .